4270
Effects of Working Memory Training on Microstructural Brain Changes in HIV-positive and Seronegative Subjects1Department of Medicine, University of Hawaii John A. Burns School of Medicine, Honolulu, HI, United States, 2Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States
Synopsis
Microstructural brain changes before, 1-month and 6-months and after working Memory Training was evaluated in both HIV-positive and seronegative individuals. While working memory training improved performance in both trained and non-trained working memory tasks, brain diffusivities increased in most brain regions after training in both groups, more in HIV than controls in some regions. These findings suggest ongoing brain inflammation associated with normal aging or HIV may mask the training-related changes in DTI measures.
INTRODUCTION
Working memory (WM) training improves performance in attention and WM, and the training effects have been associated with brain changes on structural MRI1,2 and functional MRI.3 A recent study shows that WM training also benefits HIV patients;4 however, whether improvements in brain microstructures also occur unknown. The current study aims to evaluate whether brain microstructural changes occur 1-month and 6-months after WM training using diffusion tensor imaging (DTI) in HIV-positive (HIV+) and seronegative (SN) subjects.METHODS
61 participants [33 SN: ages 52.21±2.12 years, 28(84.8%) male; 28 HIV+: ages 53.02±2.19 years, 27(96.4%) male] fulfilling study criteria completed an adaptive WM training program (Cogmed RM®, 30-45min/session; 20-25 sessions; 5-8 weeks) and were scanned before and after training (baseline, 1 and 6 months after training) at 3-Tesla (Siemens TIM Trio). Structural MRI to exclude brain pathology were acquired; these included a sagittal 3-D magnetization-prepared rapid gradient-echo (MP-RAGE) (TR/TE/TI=2200/4.11/1000ms; 1 average; 208x256x144 matrix) and fluid-attenuated inversion recovery (FLAIR) scan (TR/TE/TI = 9100/84/2500ms). DTI protocol includes b=0 and 1000s/mm2, 12 directions, TR/TE=3700/88ms, resolution 1.7x1.7mm2, 4mm axial slices with 1mm gap, 4 repetitions. DTI metrics were assessed in 9 WM-associated regions in MRIStudio (https://www.mristudio.org), using Large Deformation Diffeomorphic Metric Mapping and the JHU-MNI atlas. Fractional anisotropy (FA), mean, axial, and radial diffusivity (MD/AD/RD) were assessed in each region.5,6 The Cogmed program provided an Index of improvement after training, while non-trained WM was also assessed using Digit-Span-Forward, Backward and Sequencing, Letter-Number Sequencing and Spatial-Span-Forward and Backward at all three time points. Mixed model repeated-measures ANCOVAs (covaried for age and socioeconomic status) were performed to evaluate group differences in trained and non-trained WM task performance and DTI metrics across time points.RESULTS
Participant Characteristics: SN and HIV+ subjects were similar in age, sex, years of education, and full-scale intelligence quotient. They also had similar distributions of race and ethnicity and were predominantly white or mixed race. The average duration of HIV diagnosis was 210±21 months, mean CD4+ count was 523±51/mm3, and nadir CD4+ count was 243±31/mm3. Compared with SN controls, the HIV+ participants had lower Karnofsky Score (91.43±2.34 vs. 99.26±0.51, p=0.002) but similar scores on HIV Dementia Scale and Epidemiological Scale-Depression (CES-D).
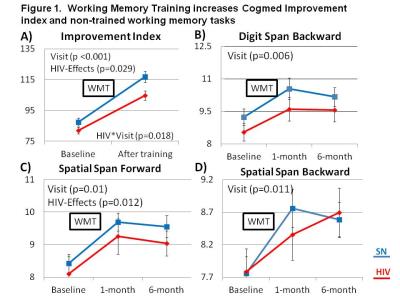
WM Training Effects: On the Index of improvement, both HIV and SN subjects improved after Cogmed training, but HIV subjects showed less improvement than SN (Figure 1A). Furthermore, although all subjects showed improvement on non-trained WM tasks after Cogmed, HIV subjects showed lower performance than SN on Digit-Span-Backward, and Spatial-Span-Forward and Backward across all three time points (Fig. 1B-D).
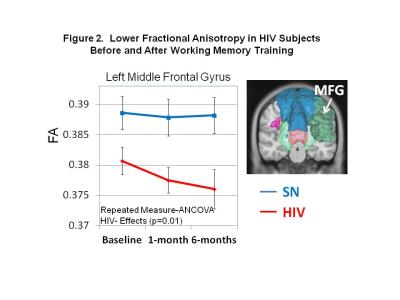
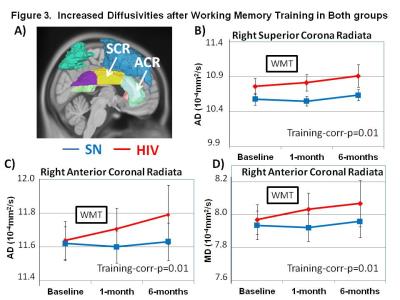
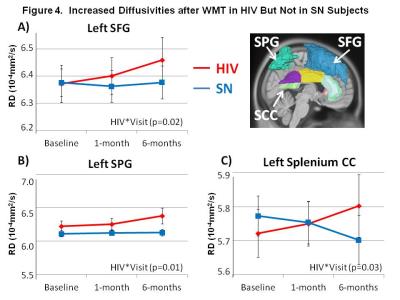
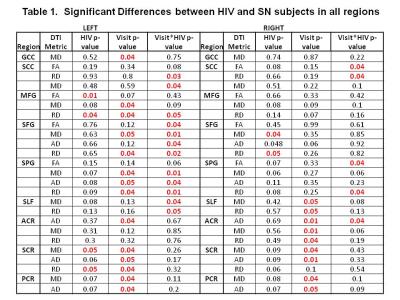
DTI Measures: HIV subjects showed persistently lower FA than SN in the left medial frontal gyrus (MFG) across time points (p=0.01; Figure 2). However, after WM training, both groups showed increased axial diffusivities in right superior corona radiata (SCR) and right anterior corona radiate (ACR), as well as mean diffusion in right ACR (Figure 3A-D). Increased diffusivities after training were also observed in other regions, predominantly in the left hemisphere (Table 1). Furthermore, HIV but not SN subjects showed increased RD (p=0.02) and MD (p=0.01) in the left superior frontal gyrus (SFG), and RD & MD (p=0.01 for both) in the left superior parietal gyrus (SPG) (Figure 4A & B). Lastly, RD increased in the splenium of the corpus callosum in HIV+ subjects but decreased in SN subjects 6-months after WMT (Figure 4C). Several other regions showed similar group-by-training effects on RD or AD, with only HIV subjects showing increased diffusivities after training (Table 1).
DISCUSSION
Consistent with prior studies,3,4 WM training improved both trained and non-trained WM tasks in all participants. Our HIV+ subjects had minimal microstructural abnormalities since they showed significantly lower FA, suggesting axonal loss, only in MFG, and increased diffusivity, consistent with neuroinflammation, only in SFG and SCR. Most brain regions showed increased diffusivities 6-months after training across all subjects; however, HIV subjects showed even greater increases in diffusion than SN in many brain regions. These findings are consistent with prior longitudinal studies that showed greater than normal age-dependent increases in brain diffusivities in HIV patients7 or decreased FA in several brain regions after inoculation with SIVsmmFGb virus.8 The decreased radial diffusion in the splenium of the corpus callosum in our SN controls after WM training is consistent with prior studies that found decreased diffusivities in healthy controls after WM training.9,10 In contrast, our HIV subjects did not show similarly decreased diffusivity or increased FA after WM training, which may be due to ongoing HIV-associated neuroinflammation,7,11 leading to much greater increases in diffusivity that might have masked any training-related decreases in diffusivities.Acknowledgements
This work was supported by the National Institutes of Health grants (1R01-DA035659; 2K24-DA16170; K02-DA16991; 1U54-NS56883; G12 MD007601). We thank Pearson Education, Inc. for providing research support for the use of the computer training program (Cogmed®). We are grateful to our research participants and the referral physicians from our community providers, including Dr. Drew Kovach, Dr. Dominic Chow, Dr. Jennifer Frank, Dr. Cyril Goshima, and the personnel at the Life Foundation, the Gregory House and at Save the Food Basket. We also appreciate the meticulous and hard work from the additional clinical and technical research staff members who were involved in the data collection for this study.References
1. Lampit, A., et al. Front Aging Neurosci. 2015, 7(14):1-14.
2. Vermeij, A., et al. Neuropsychological Rehabilitation 2016, Oct;26(5-6):783-809.
3. Klingberg, T. Trends in Cognitive Sciences 2010, 14(7):317-324.
4. Ceritoglu, C., et al. Neuroimage 2009, 47(2):618-627.
5. Oishi, K., et al. Neuroimage 2009, 46(2):486-499.
6. Chang, L., et al. Ann Neurol. 2016 Oct 19. doi: 10.1002/ana.24805. [Epub ahead of print]
7. Chang, L., et al. JNIP 2008, 3(4):265-274.
8. Li et al, Neuroimage. 2011 Sep 1;58(1):286-92. doi:
10.1016/j.neuroimage.2011.05.068.
9. Lovden, M., et al. Neuropsychologia 2010, 48(13):3878-83.
10. Strenziok, M., et al. Neuroimage 2014, 85 Pt 3:1027-39.
11. Saylor, D., et al. Nat Rev Neurol 2016, 12(4):234-48
Figures