4269
High Resolution Magnetic Resonance Histology of the Human Brain Temporal Lobe1Center for InVivo Microscopy, Department of Radiology, Duke University Medical Center, Durham, NC, United States, 2Department of Neurology, Duke University Medical Center, Durham, NC, United States, 3Departments Psychiatry and Behavioral Science, Duke University Medical Center, Durham, NC, United States
Synopsis
Detecting early brain changes in neurodegenerative diseases such as Alzheimer’s disease (AD) is essential for enabling interventions. We thus need to increase our ability to accurately localize areas that change, and to quantifying changes. The temporal lobe is essential for memory function. This is where AD hallmarks such as plaques, tangles, and neuronal death happen first. White matter has been proposed to have a role in early AD. We use high resolution magnetic resonance histology and diffusion tensor imaging to characterize the temporal lobe and its tracts. A compressed sensing acquisition with cluster based reconstruction increased efficiency four-fold.
Introduction
Understanding the changes happening in the medial temporal lobe of the brain (MTL) is essential in studies of aging and Alzheimer’s disease because of its crucial role in memory and integrating information from association areas. In addition, it is also involved in processing emotion and olfaction. The entorhinal, perirhinal, and hippocampal areas are essential structural components of the MTL. Consequently, it is important to increase the accuracy in defining the anatomical domains of the MTL and to assess structural and functional changes associated with pathology. Here we propose to use high-resolution magnetic resonance histology and diffusion tensor imaging to help enhance our understanding of the structural characteristics of the human temporal lobe.Methods
An intact fresh-frozen temporal lobe was obtained from the Bryan Alzheimer’s Disease Research Center brain bank at Duke University. The tissue specimen was then immersion fixed in 10% formalin with 0.5 mM concentration Gadoteridol (ProHance, Bracco Diagnostics, Monroe, NJ), then rehydrated for one week in 0.1M PBS with 2.5 mM Gadoteridol. The fixed and stained specimen was then immersed in liquid perfluorcarbon (Galden, Solvay Plastics, Brussels, Belgium) and scanned at 7.1T (Magnex Scientific, Yarnton, Oxford, UK) equipped with 650 mT/m Resonance Research gradient coils (Resonance Research, Inc., Billerica, MA, USA), and controlled by an Agilent VnmrJ 4.0 imaging console). RF transmission and reception relied on a 65-mm diameter quadrature RF coil (M2M Imaging, Cleveland, OH). Two imaging protocols were used to scan the temporal lobe. A multi-echo GRE sequence with FOV 80x60x60mm, matrix 800x600x600, 4 echoes, minimum TE=4.24ms, echo spacing 6.36 ms, TR =50ms, BW=155 kHz, resulting in 100-μm resolution was acquired in 5 hours. A modified 3D diffusion-weighed spin-echo pulse sequence with compressed sensing (CS) acceleration factor of 4 was also acquired with 31 unique diffusion directions and 3 non-diffusion-weighted (b0) scans. The same FOV was used as before, matrix of 400x300x300, repetition time (TR) was 100 ms, echo time (TE) = 17.01 ms. The maximum b-value was 1500 s/mm2, diffusion pulse amplitude was 48.5 G/cm, duration 5.4 ms, separation 8.9 ms. The spatial resolution was 200-μm isotropic, and acquisition time was 21h 25 min. Image reconstruction was done using pipelines implemented into a high performance computing cluster, using wavelets and total variation (1). Diffusion tensor reconstruction was done as in (2) using a pipeline which first performs eddy current corrections, and registers all DWI images to the first using an affine transform. DTI parametric images were calculated. Deterministic tractography was done using DSI Studio (3) using whole brain seeding, through the whole specimen, and the segmented hippocampal region. We chose FA thresholds 0.1-0.3, angular threshold 60°, 200000 tracks, 20% smoothing.Results
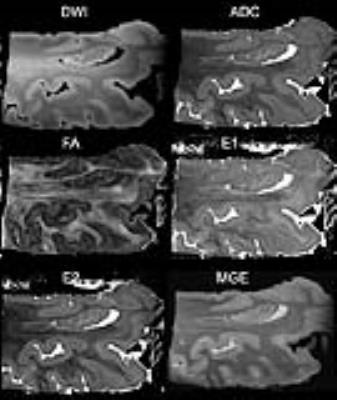
In order to obtain a multi parametric characterization of tissue changes for the temporal lobe in aging brains, in relationship with AD, we have developed two high resolution imaging protocols based on MR histology of stained specimens, which require 26 h 25 min. DTI acquisition time was 1/4 of the fully sampled k-space alternative. MR histology of the Gd-stained temporal lobe showed good contrast between white and gray matter, and details of the hippocampal layers or fields (Figure 1).
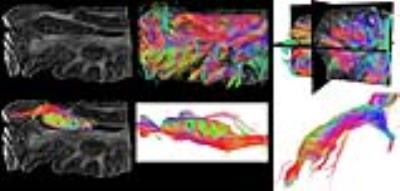
Tractography through the temporal lobe reveals a dense network of projections (Figure 2, top row), and that through the hippocampal region reveals the laminar structure of the projections through the hippocampus, at an unprecedented resolution (Figure 2, bottom row).
Discussion
MR histology provides an intermediate translation step between in vivo MR imaging of the temporal lobe, and traditional histology. The in vivo imaging of this area is challenging at high field, and resolution is limited. The high resolution obtained by classical histology in plane is often not complemented by similar resolution between planes. Obtaining high quality histological images in 3D is an attractive addition to traditional histology for :1) spatial mapping of cyotarchitectural domains, while 2) understanding the 3D spatial distribution of microstructural changes in neurodegenerative conditions, and 3) adds information to complement in vivo findings in aging and neurodegenerative conditions from population studies. White matter microstructure characterization based on DTI can give insight into the role of white matter pathology in AD, but takes a long time to provide sufficient spatial and angular resolution to resolve tracks relevant to AD. A compressed sensing acquisition implementation with a computing cluster based reconstruction alleviates these requirements, and provides sufficiently high contrast in this data set to allow definition of regions of interest such as the hippocampus, and examine relevant pathways within the temporal lobe.Acknowledgements
The brain tissue was obtained from the Bryan Alzheimer's Disease Research Center (ADRC) at Duke University. Imaging was done at the Center for In Vivo Microscopy, which is supported through NIH awards P41 EB015897 and 1S10OD010683-01 (G Allan Johnson). We also gratefully acknowledge NIH support for our research through K01 AG041211 (A Badea).References
(1) Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. MRM 2007; 58(6):1182-95.
(2) Calabrese E, Hickey P, Hulette C, Zhang J, Parente B, Lad SP, Johnson GA. Postmortem diffusion MRI of the human brainstem and thalamus for deep brain stimulator electrode localization. Hum Brain Mapp 2015; 36(8):3167-78.
(3) Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WY. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 2013; 8(11): e80713.
Figures