4266
Human Connectome Project (HCP) Lifespan Pilot: age-course of structural, microstructural and functional parameters in the hubs of the default mode network1Museo Storico della Fisica e Centro Studi e Ricerche “Enrico Fermi”, Rome, Italy, 2Center for Magnetic Resonance Research, Dept. of Radiology, University of Minnesota, Minneapolis, MN, United States, 3Division of Biostatistics, University of Minnesota, Minneapolis, MN, United States
Synopsis
Age-courses of multiple MRI outcomes were here characterized with a specific focus to default mode network (DMN) regions. Data were collected with unprecedented sensitivity and spatial resolution using the Human Connectome Project Lifespan Pilot protocol from 65 subjects divided in 4 age-groups (teen, young, middle-age and older adults). Age-related decreases of grey matter volumes, mean diffusivity, amplitude of resting-state oscillations and regional homogeneity were observed in both anterior and posterior DMN, and were more pronounced in anterior than in posterior DMN. Connectivity between posterior and anterior DMN regions remained relatively stable during the lifespan.
Purpose
The lifespan Human Connectome Project (HCP)1 aims at characterizing the development and aging of the human brain by utilizing multiple MRI outcomes with unprecedented sensitivity and spatial resolution. The goal of the present work was to use data from the HCP Lifespan Pilot study for describing the age-course of structural and functional parameters obtained with structural MRI, diffusion weighted imaging for tractography (dMRI) and resting-state functional MRI (rsfMRI). We specifically focused on two major hubs of the default mode network (DMN), namely the anterior DMN (aDMN) and posterior DMN (pDMN). The DMN is a critical network due to its link to cognitive and self-awareness functions.2Methods
MRI outcomes were obtained from a group of 65 subjects (27M/38F) who underwent the HCP Lifespan Pilot protocol at 3 T. Subjects belonged to 4 age-groups: teen (n=14, 8M/6F, 15.1±0.8 y.o.); young (n=18, 8M/10F, 29.8±3.2 y.o.); middle-age (n=18, 8M/10F, 48.9±3.3 y.o.); older adults (n=15, 3M/12F, 71.1±2.5 y.o.). The imaging protocol included MPRAGE (TE/TR=2.22/2400 ms, TI=1000 ms) and T2-SPACE (TE/TR=563/3200 ms) at 0.8 mm isotropic resolution; multiband dMRI (MB4, TR/TE=3222/89.2 ms, 1.5 mm isotropic voxels, 92 slices, 184 diffusion weighting directions, b=1500 and 3000 s/mm2) for estimates of fractional anisotropy (FA) and mean diffusivity (MD); multiband gradient-recalled echo EPI (MB8, TR/TE =720/37 ms, 2.0 mm isotropic voxels, 72 oblique-axial slices) for estimates of resting-state activity and connectivity. Data analysis followed pipelines established by the HCP and available via the GitHub repository (http://humanconnectome.org/documentation/HCP-pipelines/), including the FIX fMRI denoising and Diffusion Pipelines.3-5 Structural parameters included grey matter (GM) volume, white matter (WM) volume, and brain parenchyma fraction (BPF). Microstructural parameters included T1w/T2w ratio, FA and MD. Resting-state functional parameters included region-to-region connectivity, power spectrum and fractional amplitude of low frequency fluctuations (fALFF), and connectivity of each voxel with its neighbors, also known as regional homogeneity (ReHo). Age-group comparisons were performed with two-tailed t-tests. Framewise displacement was used as nuisance covariate in resting-state comparisons to take into account variability in head movements.Results
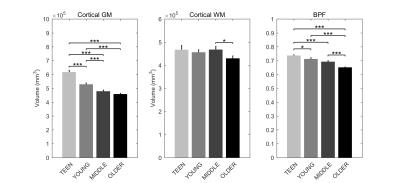
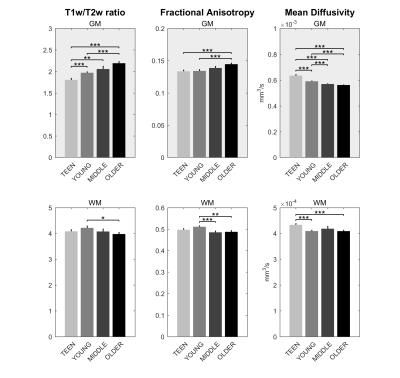
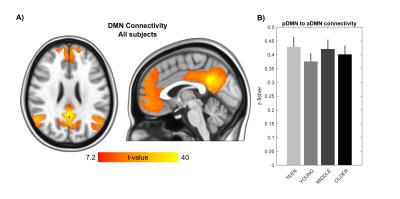
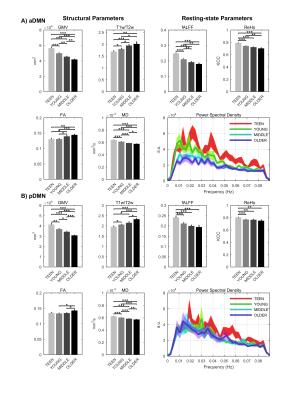
Signs of structural differences with age were observed in the whole brain (Figure 1), including reductions of cortical GM volumes and BPF, whereas the cortical WM volume was somewhat more preserved during the lifespan. Microstructural changes occurred especially in the grey matter (Figure 2). In particular, consistently lower MD and higher T1w/T2w ratio were observed across the entire investigated lifespan, whereas higher grey matter FA was observed only starting from the young age group. The rsfMRI seed-analysis with the seed in the posterior cingulate cortex (PCC) revealed the typical DMN (Figure 3). No significant changes of connectivity between pDMN and aDMN were detected during the lifespan. On the other hand, age-related differences in structural, microstructural and the other functional resting-state parameters were observed in both pDMN and aDMN during the entire lifespan (Figure 4). The lower grey matter MD, power spectrum, fALFF and ReHo, and the higher FA were more pronounced in aDMN as compared to pDMN.Discussion
Progressively lower grey matter volumes and MD across the lifespan were in agreement with previous reports.6 Interestingly, MD in the GM was strongly correlated with GM volume (r=0.68, p<10-10), perhaps indicating that the observed microstructural changes highlight substrates of macrostructural changes. The T1w/T2w ratios are commonly considered as a myelin marker.7 However the origin of the age-related T1w/T2w increase in the GM is unclear, and is likely due to a factor which shortens T2 (e.g., iron accumulation) rather than myelin change. The reduced age-associated resting-state activity in both aDMN and pDMN occurred in the presence of age-related structural and microstructural changes, but did not result in reduced connectivity between the two hubs. Importantly, all resting-state parameters were strictly calculated only in GM voxels as identified by the segmentation of each subject, and therefore were not biased by the GM atrophy that also occurs with age. Yet, there was a strong correlation between GM volumes and functional parameters (GMV:fALFF r=0.66 p<10-9, GMV:REHO r=0.58 p<10-7), although causality cannot be established based on these findings only. Further studies with larger cohort of subjects will allow identifying the age-courses of structural and functional brain parameters with more refined time-granularity.Conclusion
The lifespan HCP collects a variety of structural and functional brain properties with unprecedented sensitivity, thus leading to a deeper understanding of how the human brain develops and ages. Based on the present data obtained with the lifespan HCP piloting protocol, aging may impact the aDMN faster and more profoundly than the pDMN, however the connectivity between these two brain regions remains relatively unaffected in healthy aging.Acknowledgements
This work was supported by Supplement U54 MH091657, U01AG052564, U91MH109589, P41 EB015894, P30 NS076408. This project has also received funding from the European Union's Horizon2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 691110 (MICROBRADAM).References
1. Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, W.U.-M.H. Consortium. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62-79.
2. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253-258.
3. Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449-468.
4. Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232-247.
5. Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TE, W.U.-M.H. Consortium, Advances in diffusion MRI acquisition and processing in the Human Connectome Project. NeuroImage. 2013;80:125-143.
6. Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev. 2014;24(3):271-289.
7. Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597-11616.
Figures