4260
Brain morphological alterations and functional changes during visually stimulated sexual arousal in menopausal women1Research Institute of Medical Imaging, Chonnam National University Medical School, Gwangju, Korea, Republic of, 2Department of Radiology, Chonnam National University Hospital
Synopsis
The aging process and menopausal transition are important factors in sexual dysfunction of menopausal women. Until now, it has been unknown how menopause synchronously influences brain morphology and brain function during visually stimulated sexual arousal in menopausal women. We used structural and functional magnetic resonance imaging (fMRI) in parallel to evaluate menopause-related brain morphological and functional alterations during visually stimulated sexual arousal in menopausal women.
Introduction
Menopausal women have a variety of sexual problems including low sexual desire, difficulty with vaginal lubrication, and the inability to climax; these problems significantly impact the quality of life.1-3 Sexual dysfunction in menopausal women is 2.3 times higher compared with premenopausal women.4 Estrogens have an important neural growth and trophic effect that can be re-expressed in the adult brain in response to tissue damage.5 Estrogen deficiency in menopausal women leads to epithelial thinning and reduced lubrication of the genitalia during sexual arousal, which causes vaginal dryness and dyspareunia.6 The effects of estrogens on brain structure have been seldom investigated using a voxel-based morphometry (VBM), while functional MRI has been increasingly used to evaluate the brain centers associated with sexual arousal in women. Morphometric and functional deficits may overlap to each other, and the combined results may suggest that brain function and gray matter volume are altered together in menopausal women.7
Methods
A total of 20 premenopausal women (mean age: 42.2 ± 7.4 years) and 20 menopausal women (mean age: 55.3 ± 2.6 years) participated in this study. Twenty menopausal women were recruited using the following criteria: 1) a menopause diagnosis based on the Stages of Reproductive Aging Workshop (STRAW) +10 and the regularity of menstrual bleeding; 2) no history of a hysterectomy or bilateral oophorectomy; 3) greater than 40 μg/ml on the follicle-stimulating hormone (FSH) levels; 4) over than 1 year after last menstrual period; 5) no history of psychiatric and neurological illnesses; and 6) no history of hormone and steroid treatment and oral contraception for 1 month before our study.
MR examination was performed on a 3T Magnetom Trio MR Scanner (Siemens Medical Solutions, Erlangen, Germany) with a birdcage head coil. All volunteers underwent functional and structural MRI. Brain functional activity was measured while viewing an erotic video. The activation maps were obtained from the contrast ‘30 sec sexual activation period versus 30 sec neutral period.’ An analysis of covariance (ANCOVA) adjusting for age was used to evaluate the differential brain activation patterns and gray matter (GM) and white matter (WM) volume alterations between premenopausal and menopausal women. MRI and fMRI data were post-processed using Statistical Parametric Mapping (SPM8) software with the diffeomorphic anatomical registration through an exponentiated Lie algebra (DARTEL) algorithm.
Results and discussion
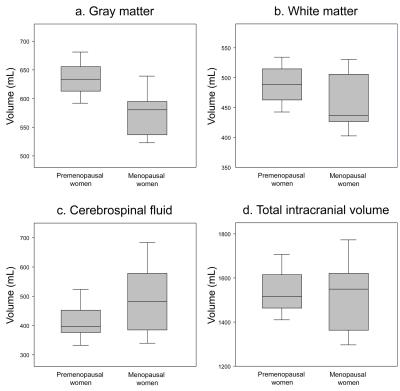
The GM volumes of premenopausal and menopausal women were 634.2±33.8 mL and 577.8±41.1 mL, respectively, whereas the WM volumes were 490.9±40.4 mL and 460.5±53.7 mL, respectively (Fig. 1). The GM and WM volumes of menopausal women were significantly lower than those of the premenopausal women (p=0.0001 for GM, p=0.027 for WM).
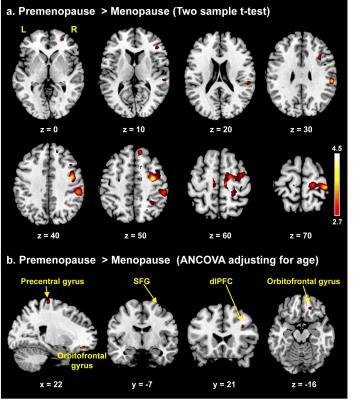
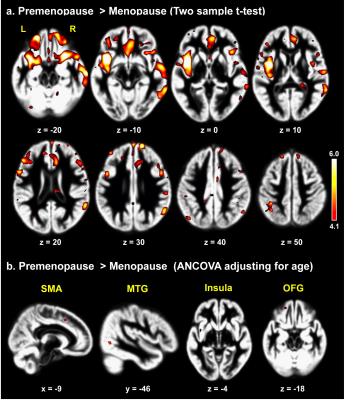
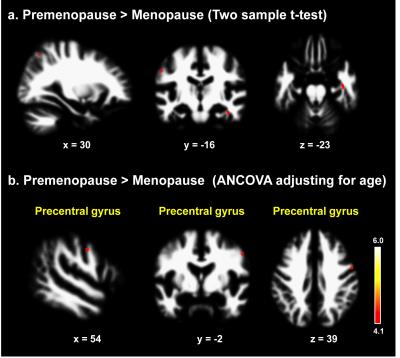
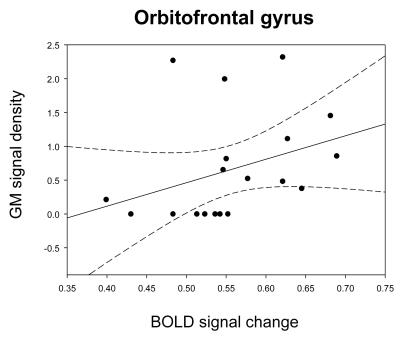
To the best of our knowledge, our study is the first to evaluate morphometric and functional alterations in menopausal women viewing sexual stimuli. Compared with premenopausal women, menopausal women showed significantly decreased activity in the precentral gyrus, orbitofrontal gyrus (OFG), superior frontal gyrus, and dorsolateral prefrontal cortex while viewing the erotic video (p<0.001, Fig. 2). In morphometric analysis, menopausal women exhibited significantly decreased GM volumes of the supplementary motor area, OFG, middle temporal gyrus, and insula and decreased WM volume of the precentral gyrus compared with premenopausal women (p<0.0001, Figs. 3, 4). In the OFG of menopausal women, we observed a reduced GM volume and decreased functional activity during together sexual arousal. In addition, blood-oxygen-level-dependent (BOLD) signal changes in the OFG during sexual arousal in menopausal women were positively correlated with the GM volume changes in the OFG (Fig. 5). Our findings suggest that decreased activation in the OFG during sexual arousal in menopausal women is closely associated with a reduction in GM volume.
Conclusion
This study revealed the effect of the combined use of functional and structural MRI on the evaluation of the association with menopause-related brain morphological and functional alterations during visually stimulated sexual arousal in women. These findings are potentially helpful in assessing the menopause-related dysfunction.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIP) (2015R1A2A2A01007827) and the Ministry of Education (2014R1A1A2006730).References
1. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. New Engl J Med. 2007;357(8):762-774.
2. Woodard TL, Nowak NT, Balon R, et al. Brain activation patterns in women with acquired hypoactive sexual desire disorder and women with normal sexual function: a cross-sectional pilot study. Fertil Steril. 2013;100(4):1068-1076.
3. Tae-Hoon Kim, Heoung-Keun Kang, Gwang-Woo Jeong, et al. Localized brain metabolite changes during visual sexual stimulation in postmenopausal women: a pilot study using functional magnetic resonance spectroscopy. Menopause. 2014;21(1):59-66.
4. Gracia CR, Freeman EW, Sammel MD, et al. Hormones and sexuality during transition to menopause. Obstet Gynecol. 2007;109(4):831-840.
5. Boccardi M, Ghidoni R, Govoni S, et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a voxel-based morphometry study. Menopause. 2006;13(4):584-591.
6. Bruce D, Rymer J. Symptoms of the menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23(1):25-32.
7. Guo W, Liu F, Yu M, et al. Functional and anatomical brain deficits in drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:1-6.
Figures