4253
Voxel-based Multiparametric Analysis of Magnetic Resonance Imaging Data for Differentiating Recurrent Glioblastoma from Delayed Radiation Necrosis1Radiology, Catholic Kwandong University International St. Mary’s Hospital, Incheon, Korea, Republic of, 2Radiology and Research Institute of Radiology, Asan Medical Center, Seoul, Korea, Republic of, 3Jeju National University Hospital
Synopsis
We evaluated a volume-weighted voxel-based multiparametric (MP) clustering method as an imaging biomarker for differentiating recurrent glioblastoma from delayed radiation necrosis, comparing to the single imaging parameters of DWI, DSC and DCE perfusion MR. In an area under the receiver operating characteristic curve analysis, volume-weighted voxel-based MP clustering demonstrated better diagnostic accuracy for discriminating these two conditions than single imaging parameters. When performed with use of an optimal cutoff, volume-weighted voxel-based MP clustering improved the overall sensitivity. Therefore, quantitative analysis using volume-weighted voxel-based MP clustering is superior to single imaging parameter measurements for differentiating recurrent glioblastoma from delayed radiation necrosis.
Purpose
To evaluate a volume-weighted voxel-based multiparametric (MP) clustering method as an imaging biomarker for differentiating recurrent glioblastoma from delayed radiation necrosis.
Methods
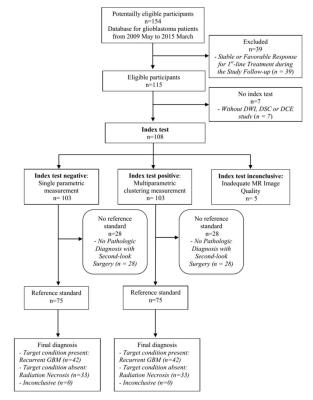
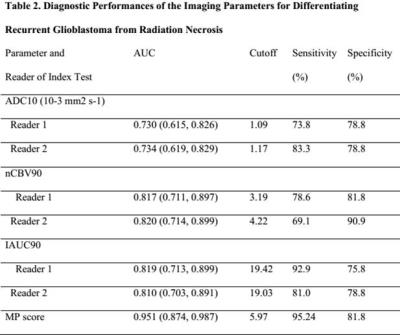
Our institutional review board approved this retrospective study and waived the informed consent requirement. Seventy-five patients with pathologically confirmed recurrent glioblastoma (n = 42) or radiation necrosis (n = 33) that presented with enlarged contrast-enhanced lesions on MR after completing concurrent chemoradiotherapy were enrolled. Using the volume-weighted voxel-based MP clustering method, the diagnostic performance of the total MP cluster score including the area under the curve (AUC), sensitivity, and specificity was compared to that of single parameter measurements (10% or 90% histogram cutoffs of apparent diffusion coefficient [ADC10], normalized cerebral blood volume [nCBV90], and initial area under the time–signal intensity curve [IAUC90]).Results
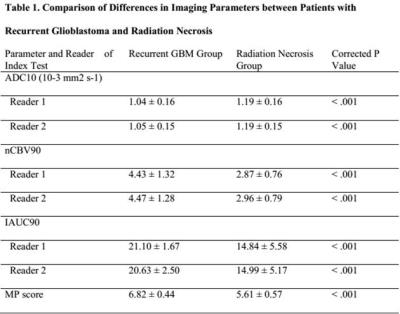
Among the 75 patients, 42 (56%) were confirmed to have recurrent glioblastoma and 33 (44%) had delayed radiation necrosis. No clinical characteristics were significantly different between the two groups. The mean value of ADC10 was significantly lower in the recurrent glioblastoma group than in the delayed radiation necrosis group (corrected P < .001 for both readers). The mean values of nCBV90 and IAUC90 were significantly higher in the recurrent glioblastoma group than in delayed radiation necrosis group (corrected P < .001 for both readers, respectively). The total MP score was also significantly higher in the recurrent glioblastoma group than in the delayed radiation necrosis group (6.82 ± 0.44 and 5.61 ± 0.57, P < .001, respectively). Receiver operating characteristic curve analysis showed that the AUC for differentiating recurrent glioblastoma from delayed radiation necrosis was highest in the total MP cluster score and lowest for ADC10 for both readers (P < .05). The total MP cluster score had significantly better diagnostic accuracy than any single parameter (P = .001–.019 for reader 1; P= .001–.017 for reader 2). Additionally, the total MP cluster score was the best predictor of recurrent glioblastoma (AUC 0.874–0.987; optimum cutoff, 5.97), with a sensitivity of 95.24% and a specificity of 81.8%.Discussion
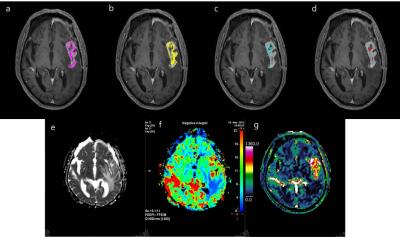
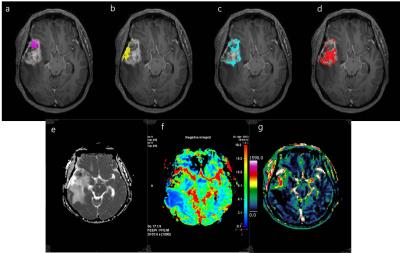
It is challenging to evaluate post-treatment glioblastoma, due to the heterogeneity of the microenvironment, including radiation necrosis, parenchymal gliosis, recurrent tumor, and inactive neoplasm1. Also, a single quantitative parameter can provide limited information reflecting one aspect of the characteristics of the lesion, such as the vascular microenvironment, or only the probability of a linear correlation between imaging parameters and the characteristics of lesions. Furthermore, the threshold value of each quantitative parameter for the discrimination of recurrent glioblastoma and delayed radiation necrosis varies between studies. A recent study suggested that a volume-weighted voxel-based MP method, which used a proper combination of quantitative parameters to reflect three different areas (predominantly viable tumor, a mix of viable tumor and treatment-related changes, and predominantly treatment-related changes) was a reliable and reproducible method for discriminating pseudoprogression from early tumor progression of glioblastoma2. In our current study, we founded that the total MP cluster score using this volume-weighted voxel-based MP method was significantly higher in recurrent glioblastoma compared to delayed radiation necrosis. Moreover, diagnostic performance using the total MP cluster score was better than that of single imaging parameters to differentiate between recurrent glioblastoma and delayed radiation necrosis in patients with enlarging contrast-enhancing lesions 12 weeks after the end of CCRT. There was also a trend toward improving diagnostic accuracy using MP clustering over single parameter. These results suggest that quantitative analysis using voxel-based MP clustering is best able to discriminate our target condition, recurrent glioblastoma, from delayed radiation necrosis, and the total MP cluster score derived from MP clustering appears superior to using single imaging parameters to predict recurrent tumor for post-treatment glioblastoma patients. Hence, this is corresponded to our intended use that the volume-weighted voxel-based MP clustering can be used as a diagnostic imaging biomarker for differentiating our target condition. Since clustering is a data-processing and image segmentation method that integrates similar data into same group and keeps clusters separate from other groups, this method may highlight characteristic values of areas predominantly containing a viable tumor, those containing a mix of viable tumor and treatment-related changes, those with predominantly treatment-related changes, thus more clearly differentiating viable tumors from radiation necrosis. Therefore, in the same context, the total MP cluster score derived from our MP clustering method was superior to measurements of single imaging parameters as a predictor of recurrent tumor for post-treatment glioblastoma patients.Conclusion
Quantitative analysis using volume-weighted voxel-based MP clustering is superior to the use of single imaging parameters for differentiating recurrent glioblastoma from delayed radiation necrosis.Acknowledgements
No acknowledgement found.References
1. Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc 1987;62(6):450-459.
2. Park JE, Kim HS, Goh MJ, Kim SJ, Kim JH. Pseudoprogression in Patients with Glioblastoma: Assessment by Using Volume-weighted Voxel-based Multiparametric Clustering of MR Imaging Data in an Independent Test Set. Radiology. 2015;275(3):792-802.
Figures