4247
Characterizing CRT-Induced Vascular Injury in the Developing Brain1Radiology & Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Neurology, University of California San Francisco, CA, United States
Synopsis
In the treatment of pediatric medulloblastomas, cranial radiation therapy (CRT) may induce long-term effects including CRT-induced vascular injury and cognitive impairments. 7T Susceptibility-weighted MRI was used to characterize CRT-induced injury in the form of cerebral microbleeds (CMBs) as potential markers of cognitive deficits. The majority of CMBs were located in the frontal lobe, which develop late in the adolescent brain. CMB density was associated with deficits in working and visual memory as a function of time since CRT. This work supports a modification of future standards for defining radiation target volumes, with evidence for early intervention with cognitive rehabilitation strategies.
Purpose
With brain tumors now the leading cause of cancer-related deaths in children under the age of 20, there is a pressing need for more research to evaluate current treatment strategies. Pediatric medulloblastomas, typically arising in the cerebellum and posterior fossa, are the most common malignant brain tumors in children. Although cranial radiation therapy (CRT) remains an integral role in the treatment of medulloblastomas, it is often associated with significant long-term side effects including vascular injury, hormonal deficiencies, and cognitive decline.1,2 Current treatment strategies have significantly improved the prognosis of medulloblastomas; the 5-year survival rate is now at 70-80% in average-risk disease.3 Thus the impact of treatment on development and quality of life are important considerations for this group, such that they can maximize their cognitive potential and go on to lead independent lives.
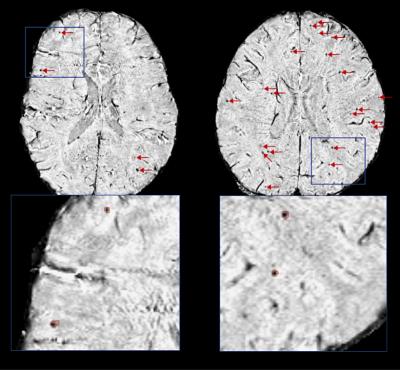
Manifestation of CRT-induced vascular injury have been detected with Susceptibility-Weighted Imaging (SWI) as early as 8 months following treatment on a 3T scanner.4 This includes hemosiderin deposits in the form of cerebral microbleeds, CMBs, which can vary in size, and/or changes in the white matter pathways and cortical thickness.4,5 Similar vascular injuries have previously been related to the cognitive decline experienced by stroke and vascular dementia patients6, however, the underlying mechanism driving CRT-associated cognitive decline still remain unclear. With the emergence of ultra-high field MR imaging offering improved visualization of vascular injury over clinical field strengths7, there is a unique opportunity to thoroughly characterize CRT-induced injury with respect to its distribution in the brain, and its relation to clinical variables, cognitive performance measures, and connectivity within cerebral networks.
Methods
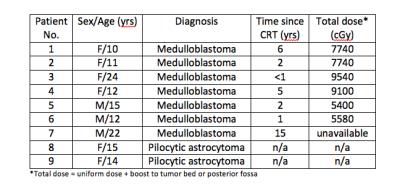
Seven patients (3 male, 4 female; mean age 15.1 ± 5.6) treated with whole-brain uniform CRT with a boost to the posterior fossa 8 months to 15 years prior for a pediatric medulloblastoma, as well as two, nonirradiated, control patients (2 female; mean age 14.5 ± 0.7) treated for pediatric low-grade gliomas (Figure 1) were scanned on a 7T scanner with a 32-channel phase array coil. High-resolution SWI data were acquired8 along with T1-weighted images of brain anatomy. Cognitive performance measures were acquired via a computerized cognitive battery (Cogstate, Newhaven, CT) designed to assess multiple domains of cognitive function. CMBs were detected using an in-house automated CMB detection algorithm9, recently updated to perform CMB volume quantification10. The distribution of CMBs was evaluated to identify regions of the brain most susceptible to CRT-induced vascular injury. Associations between CMB presence, radiation dose, time since CRT, and cognitive function were explored.Results
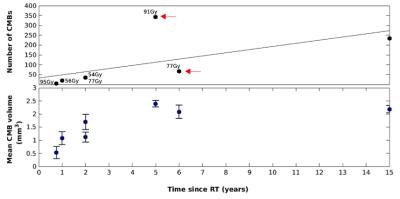
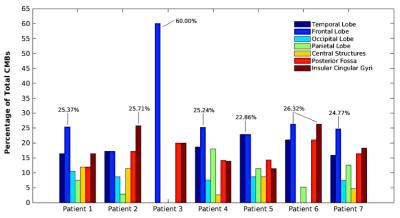
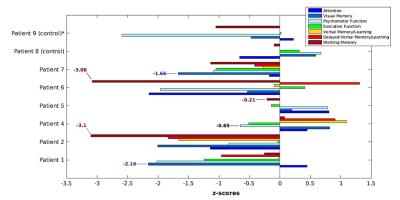
As predicted, patients in the control group did not develop any CMBs. The number and mean volume of CMBs, ranging between 5 and 342 CMBs, and 0.53 and 2.39 mm3 respectively, were positively correlated with time since CRT (Figure 2). This trend, however, was modulated by radiation dose; when comparing two patients treated 5 and 6 years prior, the patient who received a greater total dose presented with 3 times as many CMBs (Figure 3). The majority of CMBs were located in the frontal lobes, followed by the insular and cingular cortices, temporal lobes, and posterior fossa (Figure 4). Global cognitive performance scores (z-score units) were predominately worse for the irradiated patients when compared with one control (patient 8), though no correlations with CMB presence or time since CRT were observed. Domain specific scores, however, revealed similar cognitive deficits in the working memory and visual memory of patients treated either 1-2 years or > 2 years’ prior (thus having either low or high CMB density), respectively (Figure 5).Discussion/Conclusion
The existing goal of this study is to characterize CRT-induced vascular injury towards modifying future standards for defining radiation target volumes, and provide evidence for early intervention with cognitive rehabilitation strategies. Preliminary findings suggest that the effects of radiation dose with respect to CRT-induced vascular injury, become most apparent approximately 5 years post-CRT, and that the frontal lobe, which has been show to mature late in the developing brain11, is most susceptible to CRT-induced vascular injury.12 A measure of CMB density in the frontal lobe or other radio-sensitive brain areas may serve as a marker of domain-specific cognitive impairment. The present cohort augmented by four additional patients who have enrolled and consented to participate in a scan in the next 3 months, will enable a more thorough investigation of this association to determine its statistical significance. This will include an evaluation of CMB characteristics within specific brain structures, and their relation to distinct cognitive processes as well as the integrity of adjacent white matter pathways.Acknowledgements
The study was supported by NIH grant RO1HD079568.References
1. Greene-Schloesser, D., Robbins, M. E., Peiffer, A. M., Shaw, E. G., Wheeler, K. T., & Chan, M. D. (2012). Radiation-induced brain injury: A review. Frontiers in Oncology, 2(July), 73. http://doi.org/10.3389/fonc.2012.00073
2. Massimino, M., Biassoni, V., Gandola, L., Luisa Garre, M., Gatta, G., Giangaspero, F., Poggi, G., Rutkowski, S. (2011). Childhood medulloblastoma. Critical Reviews in Oncology/Hematology, 79(1), 65–83. http://doi.org/10.1016/j.critrevonc.2010.07.010
3. Medulloblastoma. American Brain Tumor Association. http://www.abta.org/brain-tumor-information/types-of-tumors/medulloblastoma.html
4. Lupo, J. M., Molinaro, A. M., Essock-Burns, E., Butowski, N., Chang, S. M., Cha, S., & Nelson, S. J. (2016). The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro-Oncology, 18(1), 87–95. http://doi.org/10.1093/neuonc/nov128
5. Valk, P. E., & Dillon, W. P. (1991). Radiation injury of the brain. American Journal of Neuroradiology, 12(1), 45–62
6. Kalaria, R. N., Akinyemi, R., & Ihara, M. (2016). Stroke injury, cognitive impairment and vascular dementia. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1862(5), 915–925. http://doi.org/10.1016/j.bbadis.2016.01.015
7. Lupo, J. M., Li, Y., Hess, C. P., & Nelson, S. J. (2011). Advances in ultra-high field MRI for the clinical management of patients with brain tumors. Current Opinion in Neurology, 24(6), 605–15. http://doi.org/10.1097/WCO.0b013e32834cd495
8. Bian, W., Banerjee, S., Kelly, D. A. C., Hess, C. P., Larson, P. E. Z., Chang, S. M., Lupo, J. M. (2015). Simultaneous imaging of radiation-induced cerebral microbleeds, arteries and veins, using a multiple gradient echo sequence at 7 Tesla. Journal of Magnetic Resonance Imaging, 42(2), 269–279. http://doi.org/10.1002/jmri.24802
9. Bian, W., Hess, C. P., Chang, S. M., Nelson, S. J., & Lupo, J. M. (2013). Computer-aided detection of radiation-induced cerebral microbleeds on susceptibility-weighted MR images. NeuroImage: Clinical, 2(1), 282–290. http://doi.org/10.1016/j.nicl.2013.01.012
10. Zou, X., Wei., B, Lafontaine, M., Hess, C.P., Nelson, S.J., Lupo, J.M. (2015). Automated 3-D Segmentation of Radiation-induced Cerebral Microbleeds on Susceptibility Weighted Imaging at 3T and 7T. International Society for Magnetic Resonance in Medicine, Toronto, 2015. Abstract #4393
11. Promise and Pitfalls of Neuroscience Research in Adolescent Health Policy. J Adolescent Health, 45(3), 216-221. http://doi.org/10.1016/j.jadohealth.2009.05.016 12. Roddy, E., & Mueller, S. (2016). Late Effects of Treatment of Pediatric Central Nervous System Tumors. J Child Neurol, 31(2), 237–254. http://doi.org/10.1177/0883073815587944
Figures