4246
Detection 2-Hydroxyglutarate in IDH-Mutant Gliomas using TE-Averaged PRESS at 3T1UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Gliomas harboring mutations in Isocitrate-Dehydrogenase (IDH) 1/2 exhibits a neomorphic-activity resulting in production of 2-hydroxyglutarate (2HG) by 2-3 orders of magnitude. Non-invasive detection of 2HG using conventional 1H Magnetic Resonance Spectroscopy (MRS) is challenging due to extensive overlap with the resonances of neighboring metabolites. Here we have designed a TE-Averaged PRESS 1H-MRS that reduces the spectral-overlaps of GABA, Gln, Glu and glutathione signals on 2HG-2.25ppm-resonance that provides a reliable-detection of 2HG. We have also estimated T2 in each brain-tumor by taking the advantage of multiple-TEs used in TE-averaging acquisitions, and used patient-specific T2 for estimation of 2HG.
Introduction
Gliomas harboring mutations in Isocitrate-Dehydrogenase (IDH) 1/2 exhibits a neomorphic-activity resulting in production of 2-hydroxyglutarate (2HG) by 2-3 orders of magnitude1,2. Noninvasive identification of elevated 2HG has gained significant clinical use in patient care3,4. Non-invasive detection of 2HG using conventional 1H Magnetic Resonance Spectroscopy (MRS) is challenging due to extensive overlap with the resonances of neighboring metabolites. The 2HG 2.25 ppm resonance is obscured by GABA-C2, Glutamate-C4 (Glu), and Glutamine-C3 (Gln) signals. Currently one of the well-established point-resolved spectroscopy (PRESS) 1H-MRS strategy for 2HG-detection employs an echo-time (TE) of 97ms3,5 which is also limited by extensive overlaps from neighboring resonances. The 2HG and interference resonances are all strongly-coupled consequently, the coherences evolve with TE in complex manners. Constructive or destructive addition of these coupled signals by averaging the signals from varied TE may help to diminish the spectral overlaps on 2HG 2.25ppm resonance. Here we have optimized a TE-Averaged PRESS 1H-MRS that reduces the spectral-overlaps of GABA, Gln and Glu and glutathione (GSH) signals on 2HG 2.25ppm resonance and provides a reliable detection of 2HG in IDH–mutant gliomas. Moreover tumors have altered inter/intra-cellular environment thus may have different Transverse-relaxation time (T2) in different tumor-types and grades which can significantly influence quantifications of metabolites. Therefore we have also estimated T2 in each brain tumor by taking the advantage of multiple TEs used in TE-averaging acquisitions, and have incorporated patient-specific T2 for estimation of metabolites.Methods
Density-Matrix simulations were performed to obtain signal-pattern and strength of major metabolites at different TEs of PRESS by varying the TE2. TE1 was fixed at 32ms. Possible combinations of number of TEs were tested to obtain a reliable 2HG upright-signal with reduced spectral-overlaps from GABA, Gln and Glu, and GSH. Optimized combination of TEs were used to obtain In-vivo spectra from Glioma patients. The protocol was approved by the Institutional-Review Board. 1H MR Experiments were carried out on a whole-body 3T scanner (Philips Medical Systems). Water-suppressed and unsuppressed water data was acquired from a FLAIR enhancing region at the optimized train of TEs from a defined voxel, using TR = 2 s, sweep width = 2500 Hz, sampling-points = 2048 and number of averages = 16 for each TE. Water signal was suppressed using a four-pulse scheme. Data was apodized with a 2-Hz exponential filter. Eddy- current-compensation and frequency-drift corrections were performed using in-house Matlab programs. Metabolite signal estimates were calculated using LCModel software. Different basis sets were prepared for each patients by incorporating T2 values of that specific tumor, using published chemical shift and J coupling constants with volume- localization-RF and gradient-pulses. Correlation significance-test was done to determine if the T2s of Cho and other measurable resonances have any relation in different tumors.Results and Discussions
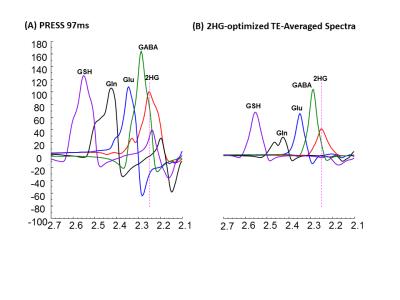
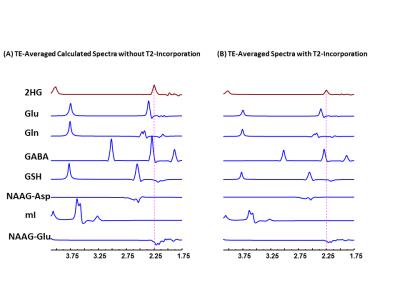
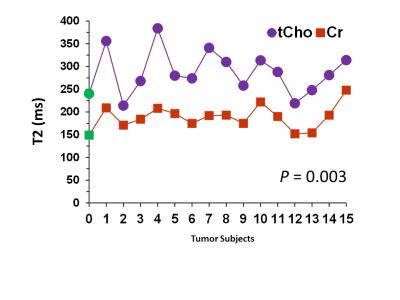
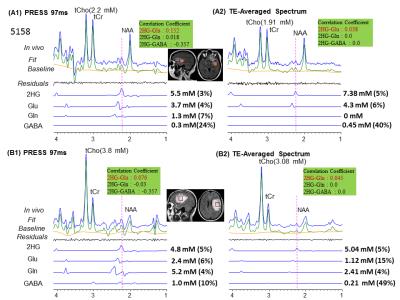
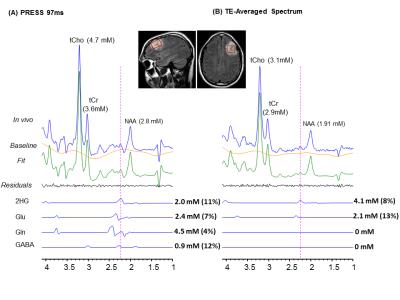
Combination of n=12 TEs with fixed TE1=32ms and variable TE2 with difference of 15ms beginning at TE2=26 ms yielded ~40%, ~39% and 30% reduction of Glu, GABA, and Gln signals compared to PRESS 97ms signal strength. TE-Averaged signal of 2HG upon incorporation of T2 of 180 ms was ~43% of that in PRESS spectra and almost had a flat baseline as the complex positive and negative overlapping signals from GSH, GABA, Glu and Gln near 2.25ppm got destructively added due to multiple-TE-averaging (Fig1). T2-incorporated (180 ms) TE-averaged signal-pattern of calculated-spectra of major coupled resonances was not different than that obtained without-T2 incorporation, but had substantially reduced signal strength (Fig.2). Monoexponential fitting of Cho, and Cr signals yielded mean Cho-T2 of 288±50 ms, and Cr-T2 of 186±20 ms across the 15-glioma patients. Cho-T2 and Cr-T2 within-tumors were found to be significantly (p=0.0003) correlated (Fig.3). TE-Averaged spectra from a Low-Grade-Glioma tumor (Fig. 4B) shows reduced 2HG-Glu, 2HG-Gln and GABA correlation-coefficients compared to TE-97ms data (Fig4 B and D) indicating the independence of 2HG signals from these-neighboring metabolites (Fig.4A) compared to PRESS-97ms estimates. Fig.5 shows TE-averaged data from an IDH-mutant Grade-2 oligodendroglioma with ~30% reduced-CRLB of 2HG compared to PRESS-97ms, and also exhibits higher 2HG estimate upon incorporation of patient-specific T2. Further, Gln and GABA signals were almost nulled in TE-averaged spectra (Fig.5B) thus indicating reliable detection of 2HG without significant contamination from the neighboring resonances compared to PRESS 97ms estimation.Conclusion
2HG-optimized TE-averaged spectra had minimal overlap and baseline distortion thus enables precise detection of 2HG. Correlation coefficients and CRLBs were smaller in TE-averaged spectra, and also Gln, GABA and GSH signals underneath 2HG were almost nulled indicating the successful application of optimized TE-averaging method for reliable 2HG estimation.Acknowledgements
National Cancer Institute of the National Institutes of Health under Award Number R01CA184584 and by a Cancer Prevention Research Institute of Texas grant RP130427.References
1. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009; 462:739-744.
2. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010; 17:225-34.
3. Choi C, Ganji SK, DeBerardinis J, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in subjects with IDH-mutated gliomas. Nat Med. 2012; 18(4):624-629.
4. Choi C, Raisanen JM, Ganji SK, et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma. J. Clinical Oncology 2016; 34(33):4030-4039.
5. Govindaraju V, Young K, Maudsley A et al. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 2000; 13:129-153
Figures