4243
Spectroscopic Imaging-based detection of 2-hydroxyglutarate (2HG) in IDH1 mutant human gliomas on 3T Clinical1Neuroradiology, University of Pennsylvania, Philadelphia, PA, United States, 2Cellular and Molecular Physiology, University of Liverpool, Liverpool, United Kingdom, 3Pathology, University of Pennsylvania, Philadelphia, PA, United States, 4Radiology, University of California Los Angeles, Los Angeles, CA, United States, 5Hematology Oncology, University of Pennsylvania, Philadelphia, PA, United States, 6Neurosurgical Oncology, University of Pennsylvania, Philadelphia, PA, United States, 7Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Mutations in isocitrate dehydrogenase (IDH) play an increasing role in clinical assessment of human gliomas and determination of treatment. The performance of Chemical Shift Imaging (CSI) to detect 2-hydroxyglutarate (2HG) in mutant-IDH gliomas was assessed in the routine clinical environment. Specificity of 80% and sensitivity of 63% was achieved in a cohort of 15 patients scanned with the technique at 3T. Greater sensitivity, through longer acqusition or more sensitive equipment could result in reliable non-invasive detection of this putative biomarker present in a majority of Grade II/III gliomas.
Purpose
Mutations in isocitrate dehydrogenase (IDH) have received greater clinical interest with the restructuring of the 2016 WHO Classification of central nervous system (CNS) tumors. These mutations, present in majorities of WHO Grade II/III gliomas (1,2), are associated with better prognosis, including longer survival and greater sensitivity to chemo-radiation therapy. IDH mutations may disrupt the usual catalysis of isocitrate into α-ketoglutarate, instead producing the “oncometabolite” 2-hydroxyglutarate (2HG) (3). Magnetic resonance spectroscopy (MRS) has emerged as a technique for reliably and non-invasively detecting this putative biomarker (4,5). Most previous research into quantifying 2HG has relied on specialized techniques like spectral editing (6) or otherwise relied on uncommon hardware like ultrahigh-field 7T scanners (7). Choi et al. (8) have previously implemented a modified echo-time (TE) Chemical shift imaging (CSI) technique at 3T in detecting 2HG. This study aims to assess the performance MRS as part of a routine clinical magnetic resonance imaging (MRI) protocol in detecting 2HG in patients harboring mutant-IDH gliomas.
Methods
Fifteen patients harboring gliomas (10 male, 5 female, mean age 57 ± 16.3 years) were scanned using on a Siemens 3T clinical MRI scanner with a 12-channel receive head coil. CSI scan parameters included TE = 97 ms, TR = 2 s, NEX = 1, 2048 t2 points with bandwidth = 2000 Hz, 16x16 resolution scan time of 6:53 min. CSI voxels 1 x 1 x 3 cm3 of were localized using T1-weighted MRI. CSI data were reconstructed offline and quantified through the LCModel prior-knowledge based fitting program to quantify the resonances of 2HG at 4.02, 2.27, 2.22, 1.98 and 1.83 parts per million (ppm). Criteria for determining IDH mutation included: presence of 2HG in multiple neighboring voxels, showing good correlation with T1 contrast or T2 fluid attenuated inversion recovery (FLAIR) imaging, and Cramer-Rao lower bounds (CRLB) ≤40%. Scanning and determination of 2HG presence was performed prospective to biopsy. A pathology subsequently performed Immunohistochemical (IHC) staining to determine IDH mutation status.Results
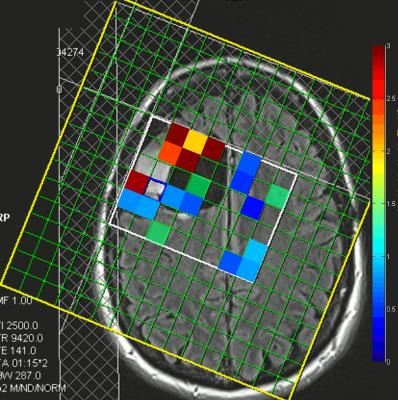
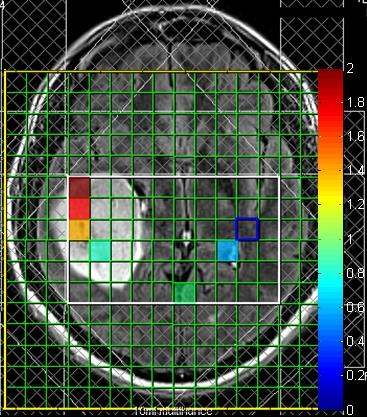
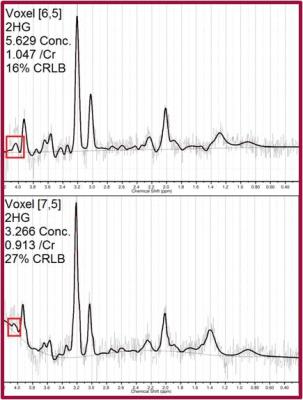
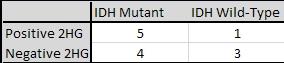
Figures 1 and 2 show quantified 2HG/Creatine (Cr) maps in patients with gliomas harboring IDH mutation. Both patients showed presence of 2HG in the area of the tumor. Figure 3 shows LCModel-fitted spectra from the same tumor shown in Figure 2 with the 4.02 ppm resonance of 2HG present in the spectra (identified by a red box). Figure 4 shows spectra localized within a wild-type IDH glioma identified as absent of 2HG showing high CRLBs and no resonance visible in the 4.02 ppm range identified by the red boxes. Table 1 shows the performance of LCModel-based quantification of 3T CSI in prospectively detecting presence of 2HG. Four of five gliomas showing presence of 2HG were subsequently identified by immunohistochemistry as mutant-IDH (80% specificity). Five of eight gliomas where presence of 2HG not identified were determined to be wild-type IDH (63% sensitivity).Discussion
LCModel quantification of 3T CSI data successfully detected 2HG, confirming previous research and suggesting the potential to non-invasively identify IDH mutation in a routine clinical MRI scanning protocol. Prospectively restricting the criteria for determining presence of 2HG to require multiple positive voxels and CRLBs ≤40% resulted in relatively high sensitivity and positive predictive values, but sensitivity lagged behind. This lower sensitivity may be a result of the lower signal quality afforded by routine clinical hardware and scan parameters. Improvements to the technique aimed at improving signal quality may include larger voxel size, more averages or a more sensitivity receiver coil could potentially facilitate higher sensitivity, though these may result in more restrictive inclusion criteria, longer scan times or greater facility costs, respectively. The improved signal quality and spectral separation facilitated by moving to higher fields may further improve the viability of this technique as ultra-high field scanners become more commonplace.Conclusion
Non-invasive detection of 2HG could improve and accelerate treatment decisions for mutant-IDH gliomas including initiation of chemo-radiation or other targeted therapies.Acknowledgements
The authors would like to acknowledge MRI technicians and coordinators at the University of Pennsylvania, including Lisa Desiderio and Katelyn O'Reilly.References
1. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ. IDH1 and IDH2 mutations in gliomas. New England Journal of Medicine 2009;360(8):765-773. 2. Ichimura K, Pearson DM, Kocialkowski S, Bäcklund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro-oncology 2009;11(4):341-347. 3. Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP+-dependent isocitrate dehydrogenase mutations. PloS one 2011;6(2):e16812. 4. Pope WB, Prins RM, Thomas MA, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. Journal of neuro-oncology 2012;107(1):197-205. 5. Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. Journal of the National Cancer Institute 2010. 6. Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4(116):116ra114. 7. Verma G, Mohan S, Nasrallah MP, Brem S, Lee JY, Chawla S, Wang S, Nagarajan R, Thomas MA, Poptani H. Non-invasive detection of 2-hydroxyglutarate in IDH-mutated gliomas using two-dimensional localized correlation spectroscopy (2D L-COSY) at 7 Tesla. Journal of Translational Medicine 2016;14(1):274. 8. Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang X-L, Mashimo T, Raisanen JM, Marin-Valencia I. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature medicine 2012;18(4):624-629.Figures