4236
Ferumoxytol Iron Nanoparticle Enhanced MR Imaging is a Noninvasive Biomarker of Isocitrate Dehydrogenase Mutational Status in Recurrent Glioblastoma and Pseudoprogression1Radiology, Oregon Health & Science University, Portland, OR, United States, 2Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States, 3Neurology, Oregon Health & Science University, Portland, OR, United States, 4Radiation Medicine, Oregon Health & Science University, Portland, OR, United States, 5Neurological Surgery, Oregon Health & Science University, Portland, OR, United States
Synopsis
Ferumoxytol iron nanoparticles are used as an off label molecular MR imaging contrast agent in patients with reduced renal function precluding gadolinium administration. Glioblastoma molecular features are now recognized as an integral component of glioma pathogenetic classification and clinical outcome. IDH1 mutation accounts for approximately 10% of glioblastoma. The absence of a reliable noninvasive biomarker of glioblastoma IDH mutation prompted this retrospective study to determine if Ferumoxytol MR Imaging is diagnostic of IDH mutational status. We observed that the presence of increased Ferumoxytol to Gadolinum enhancing ratio was a significant 3T MR imaging biomarker for IDH mutational status in recurrent glioblastoma and the differentiation of pseudoprogression.
Purpose
Glioblastoma is a uniformly fatal disease. Recent genetic molecular advances have contributed to a better understanding of glioblastoma pathophysiology. Mutations involving the IDH1 and IDH2 encoding genes have been described as glioblastoma genetic driver mutations.1-4 Currently, the diagnosis of IDH mutational status requires surgical tissue sampling. A reliable noninvasive biomarker of IDH mutation has not been previously described. Therefore, the primary aim of this retrospective study was to determine if Ferumoxytol (Feraheme, AMAG pharmaceuticals) iron nanoparticle contrast enhanced (FeCE) MR Imaging was diagnostic of glioblastoma IDH mutational status. Additionally, given the ability of FeCE to demonstrate neuroinflammation we aimed to determine if recurrent disease could be differentiated from pseudoprogression in this cohort.Methods
In this IRB-approved study, 28 patients (21 males, 7 females, mean age 57.7 years old ± 11.1 years) previously treated with maximal safe resection and temozolomide based chemoradiotherapy (CRT) following diagnosis of glioblastoma underwent gadolinium contrast enhanced (gadoteridol, Bracco) (GdCE) and then FeCE MR imaging to assess disease progression on a 3T clinical scanner (Philips Healthcare). The patients reported in this study are of a separate cohort reported by Horvath et. al.5 IDH1 mutational status was characterized by exome sequencing. FeCE was performed with T1-weighted spin echo MR imaging 24 hours following the intravenous administration of 4 to 8 mg/kg Ferumoxytol. All GdCE MR examinations were performed within 48 hours before Ferumoxytol administration. The sum of products diameter (SPD) was calculated according to RANO criteria at the tumor location for both contrast agents.6 The mismatch between enhancing lesions SPD was calculated as a ratio of FeCE to GdCE. Students t-test was used to assess for differences in SPD and FeCE/GdCE ratios between the cohorts. Receiver operator curve (ROC) analysis provided an optimal cutoff value allowing for the determination of sensitivity and specificity. P-value less than 0.05 was considered statistically significant.Results
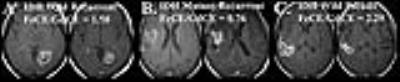
Three of the 28 patients were found to have mutated IDH1 status. Patients with IDH mutation (mean ± standard deviation, 0.92 ± 0.21) were found to have a significantly lower FeCE/GdCE SPD ratio when compared to wild type tumors (1.1 ± 0.07; P=0.01; Figure 1). ROC analysis demonstrated an optimal cutoff value of 1.00 which provided a sensitivity of 1.0 and specificity of 0.8. No significant difference was observed between measured GdCE or FeCE SPD within the IDH mutated or wild type tumors (P> 0.64). Recurrent disease was diagnosed in 25 patients (22 IDH wild type). The remaining 3 patients demonstrated clinical and radiographical evidence of pseudoprogression. All patients with pseudoprogression were IDH wild type. Patients with pseudoprogession at the time of Ferumoxytol administration demonstrated a significantly elevated FeCE/GdCE ratio (2.43 ± 0.27) when compared to IDH matched recurrent disease (1.11 ± 0.21; P= 0.01; Figure 1).Discussion
Our preliminary data suggests that, following CRT, patients with recurrent IDH wild type glioblastoma demonstrate increased FeCE/GdCE ratios when compared to IDH mutated tumors. Additionally, we observed that patients with IDH wild type pseudoprogression demonstrate markedly disproportionate elevations in FeCE/GdCE when compared to IDH matched disease recurrence. McConnell et. al. have demonstrated that FeCE is a molecular MR imaging biomarker of tumor associated neuroinflammation.7 As such, increased levels of neuroinflammation within recurrent IDH wild type glioblastoma following CRT may account for the observed increased FeCE/GdCE ratios. Interestingly, the degree of FeCE/GdCE was most prominent in patients with pseudoprogression (all greater then 2.26). These findings suggest that while treatment-induced neuroinflammation is observed within recurrent disease and pseudoprogression, it is disproportionately so within the latter. This is consistent with the current pathophysiological understanding of neuroinflammation-mediated pseudoprogression.8 When stratified by IDH mutaiton, the mechanistic differences in CRT-induced neuroinflammation within recurrent glioblastoma remains to be adjudicated. Unlike GdCE, FeCE allows for specific localization of the inflammation within brain tumors at delayed imaging time points.6 The results of this study suggest that the unique capability of iron nanoparticle contrast agents to localize sites of neuroinflammation may allow them to serve as a sensitive and specific biomarker of glioblastoma IDH mutational status. These results are clinically significant as there are currently no reliable and noninvasive imaging biomarkers of glioblastoma IDH mutational status. The validation of a quantifiable and biologically specific imaging biomarker for neuroinflammation has the potential to significantly advance the field of neuroimaging. Any hope for improving clinical outcomes lies in the development of personalized therapeutic regiments and imaging strategies. Together, these approaches could impact treatment, imaging, and survival in patients with glioblastoma.Conclusion
FeCE/GdCE mismatch provides a robust imaging biomarker capable of identifying IDH mutational status and neuroinfammation-induced pseudoprogression in patients with glioblastoma.Acknowledgements
The first author thanks the patients that participated in this study and the hard work of the staff of the OHSU Blood Brain Barrier Program.References
1. Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol. 2012 Jul;29(3):131-9.
2. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812.
3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803-20.
4. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013;19:764–772.
5. Horvath A, Varallyay C, Schwartz D, et al. Quantitative, semiautomatic comparison of ferumoxytol and gadoteridol enhancement in treated glioma patients. 2017 ISMRM submitted abstract.
6. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010 Apr 10;28(11):1963-72.
7. McConnell HL, Schwartz DL, Richardson BE, et al. Ferumoxytol nanoparticle uptake in brain during acute neuroinflammation is cell-specific. Nanomedicine. 2016 Aug;12(6):1535-42.
8. Brandsma D1, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008 May;9(5):453-61.
Figures