4229
Altered Hippocampus Microstructure in Schizophrenia: A Diffusional Kurtosis Imaging and Magnetic Resonance Spectroscopy Imaging Study1Radiology, New York University School of Medicine, New York, NY, United States, 2Psychiatry, New York University School of Medicine, New York, NY, United States
Synopsis
Diffusional Kurtosis Imaging (DKI) and Magnetic Resonance Spectroscopy Imaging were employed to assess microstructural changes of hippocampus in patients with schizophrenia. Increased mean kurtosis and mean diffusivity were observed for both left and right hippocampi in patient compared to the control group. In patients, mean kurtosis showed a strong negative correlation with N-acetylaspartate concentration. These results suggest disorganized hippocampal microstructure, likely due to neuroinflammatory processes such as micro- and astrogliosis and/or disorganized neuronal domains. DKI’s apparent sensitivity to microstructural deficits may ultimately be employed to identify individuals with microscopic hippocampal impairment.
Introduction
Hippocampal abnormalities are assumed to play a central role in the pathology of schizophrenia1. Whereas both structural and metabolical abnormalities have been reported to date2,3, the driving underlying mechanisms are poorly understood, which impedes development of targeted treatments that may alleviate core deficits of the disorder. In this study we examined microstructural properties of the hippocampus in schizophrenia patients versus comparison healthy controls using diffusional kurtosis imaging (DKI), and its associated metrics, mean kurtosis (MK) and mean diffusivity (MD). We also investigated association between diffusion metrics and metabolical concentrations of N-acetyl aspartate (NAA), creatine (Cr), and choline (Cho) in a subgroup of patients with schizophrenia, for whom Magnetic Resonance Spectroscopy Imaging (MRSI) of the hippocampus was also performed. The goal of the study was to employ a multimodal imaging approach to advance the understanding of hippocampal alterations in schizophrenia.Methods
DKI data was collected in eighteen patients with a diagnostic of schizophrenia and eighteen healthy comparison controls using a Siemens 3T Trio scanner. All participants were right-handed males with ages between 30 and 55 years old. The DKI acquisition included collection of 128 diffusion encoding directions distributed across two shells (b=1000 and b=2000 s/mm2) and of ten image volumes with b=0s/mm2. DKI data was acquired with a voxel size of 2.3x2.3x2.3 mm3. MRSI data were also obtained for six of the patients for a volume of interest (VOI) aligned and encompassing the bilateral hippocampi. The hippocampus VOI was then excited using point resolved spectroscopy with TE= 35 ms and TR=1400 ms, an acquisition matrix of 16x16x4, and a voxel size of 1.0×1.0×0.5 cm3. In addition, an anatomical T1-weighted MPRAGE image was obtained and employed to segment the right and left hippocampi. The segmented volumes were employed to calculate hippocampal volumes and were also registered to the DKI and MRSI spaces and used as regions of interest in the analyses. The DKI data was employed to obtain mean hippocampal MK and MD. The MRSI data was employed to obtain mean hippocampal NAA, Cho, and Cr. Comparison between volumetric and diffusion properties of hippocampus in patients versus controls were conducted using t-tests. Associations between diffusion and spectroscopy metrics were examined using two-tailed Pearson correlations.Results
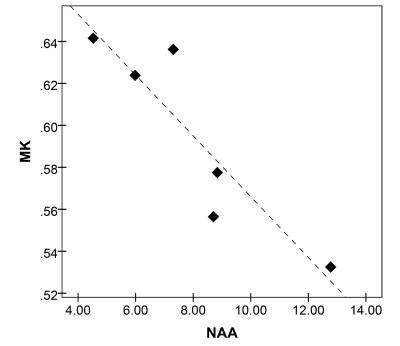
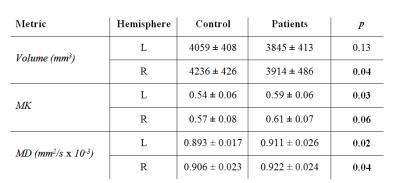
MK and MD were increased and volumes were decreased for both right and left hippocampus (Table 1 and Figure 1). MK showed a strong negative correlation with NAA concentration (R = - 0.91, p=.01) as shown in Figure 2. No significant correlations were found between either MK or MD and either of Cho or Cr concentrations.Discussion
These results support previous reports on disorganized hippocampal microstructure and decreased hippocampal volumes in schizophrenia and further suggest two potential mechanisms of dysfunction. First, inflammation may act as a common pathological substrate of both increased MK and MD, and decreased NAA. This view is supported by recent findings of increased MK and MD in areas of inflammation in animal models of traumatic brain injury5 and stroke6. Inflammation may also explain the observed MK-NAA association as inflammatory mediators released by activated glia may be neurotoxic and lead to neuronal injury, dysfunction, or neuronal loss, which are reflected by decreased NAA4. Second, disordered neuronal domains, previously observed in schizophrenia, may also lead to increased MK and denote neuronal dysfunction and decreased NAA. We did not see a strong correlation between MK or MD and Cr or Cho concentration, which suggests that the processes that dramatically affect Cr and Cho (i.e. dysmyelination, tissue death) are less likely to contribute to the microstructural changes of hippocampus in patients with schizophrenia.
Conclusions
The DKI approach, which provides compared to DTI, mean kurtosis (MK) as an additional microstructural metric to describe tissue complexity, offers a more nuanced description of the microstructural deficits of the hippocampus in schizophrenia. MRSI provides complementary information that further supports neuroinflammation and/or disorganized neuronal domains as primary candidate mechanisms of hippocampal dysfunction in schizophrenia. Better defining the disease mechanisms at tissue level may lead to more precise treatments to ameliorate deficits. Further studies will explore associations between DKI and MRSI markers in larger samples across both patient and control populations.Acknowledgements
We acknowledge grant awards from the National Institutes of Health for supporting this work.References
1. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry 2010; 167:1178-1193.
2. Chiapponi C, Piras F, Fagioli S, Girardi P, Caltagirone C, Spalletta G. Hippocampus age-related microstructural changes in schizophrenia: a case-control mean diffusivity study. Schizophr Res., 2014; 157:214-217.
3. Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. Journal of neurochemistry, 2014; 128:1-87.
4. Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 2013; 8:576-593.
5. Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage, 2012; 59:467-477.
6.Umesh Rudrapatna S, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, et al. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. Neuroimage, 2014; 97:363-373.
Figures