4228
Evaluation of abnormal structural changes in major depressive disorder with self-harm using generalized q-sampling MRI1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2School of Medicine, Chang Gung University, Taoyuan, Taiwan, 3Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan, 4Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
Major depressive disorder (MDD) is a significant brain dysfunction that might cause self-harm behavior. The abnormal brain structures between MDD and healthy control have been investigated by using diffusion tensor imaging (DTI) in several studies. However, few studies discussed the brain structure changes in MDD patients with self-harm behavior. Moreover, there were some limitations in DTI. Therefore, our study aimed to find the abnormalities of neurological structure of white matter among MDD without self-harm behavior (MDD_N), MDD with self-harm behavior (MDD_S), and healthy control (HC), using generalized q-sampling imaging (GQI). We found the significant differences in the corpus callosum, superior longitudinal fasciculus (SLF), cingulum, and frontal lobe of GQI indices in individual groups.
Introduction
Major depressive disorder (MDD) is a significant brain dysfunction that might cause self-harm behavior. Although there are several studies using diffusion tensor imaging (DTI) to find the different fractional anisotropy (FA) between MDD patients and healthy control1-3, few studies discussed the brain structure changes in MDD patients with self-harm behavior. Moreover, there are some limitations in DTI, such as it cannot recognize the complex crossing or branching patterns of white matter tracts. Therefore, our study aimed to find the abnormalities of neurological structure of white matter among MDD without self-harm behavior (MDD_N), MDD with self-harm behavior (MDD_S), and healthy control (HC) using generalized q-sampling imaging (GQI).Methods
All participants, including 28 MDD_N, 30 MDD_S, and 28 HC, were arranged for a brain GQI examination on 3T MRI (Verio, Siemens, Germany). The GQI parameters included TR/TE = 8943/115 ms; 35 axial contiguous slices; voxel size = 1.7 x 1.7 x 4 mm3; FOV=220 x 220 mm2; 193 diffusion directions with b-values of 1000, 1500, 2000 s/mm2 and scan time was around 30 min. All raw data were corrected first with FSL (FMRIB, Oxford, UK) to reduce eddy current distortion. Subsequently, the corrected diffusion images were spatially normalized to the Montreal Neurological Institute (MNI) T2WI template using parameters determined from the normalization of the diffusion null image to the T2WI template using Statistical Parametric Mapping 8 (SPM8, Wellcome Trust Centre for Neuroimaging, UK). GQI reconstruction was performed by DSI studio (National Taiwan University, Taipei, Taiwan), and GQI indices, including normalized quantitative anisotropy (NQA), generalized fractional anisotropy (GFA) and isotropic of the orientation distribution function (ISO), were calculated. For the statistical analysis, two sample t-test was used to detect the significant differences among MDD_N, MDD_S and, HC on GQI indices. Participants’ gender and education year were used as covariates.Results
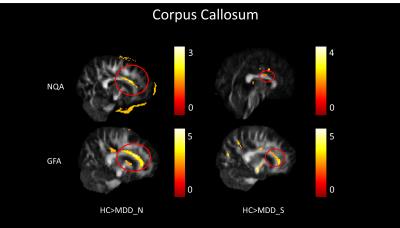
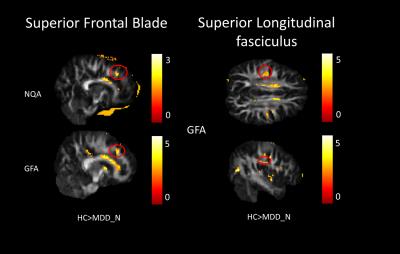
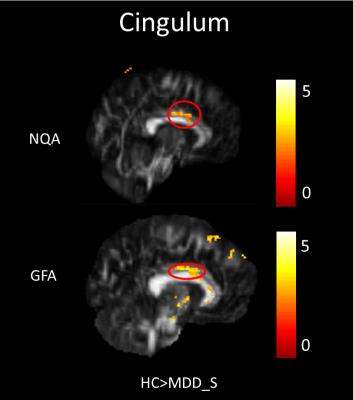
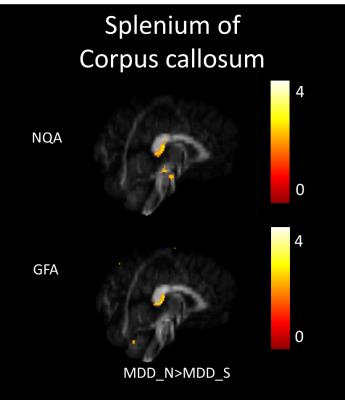
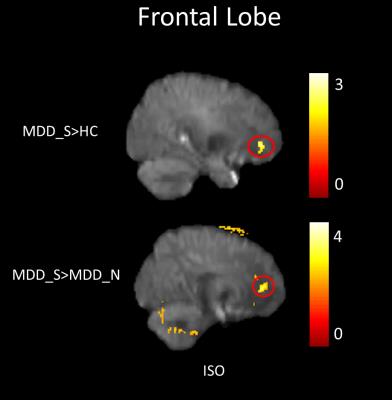
In our study, a decrease of NQA (p<0.05) and GFA (p<0.01) in the genu of the corpus callosum were found among MDD_N and MDD_S when compared with HC (Fig. 1). There was also a decrease of NQA (p<0.05) and GFA (p<0.01) in the left superior frontal blade among MDD_N compared to HC (Fig. 2). Besides, GFA (p<0.01) of MDD_N were lower than HC in the superior longitudinal fasciculus (SLF) (Fig. 2). Moreover, both NQA (p<0.05) and GFA (p<0.01) of MDD_S were lower in the left cingulum compared to HC (Fig. 3). When MDD_S was compared to MDD_N, a decrease of NQA (p<0.03) and GFA (p<0.03) in the splenium of the corpus callosum of MDD_S was found (Fig. 4). There was also an increase of ISO in the frontal lobe among MDD_S when compared to MDD_N (p<0.04) and HC (p<0.02) (Fig. 5).Discussion
Several DTI studies discovered lower FA in the cingulum, SLF, and corpus callosum among MDD when compared to HC 2, 4, 5. The cingulum is part of the limbic system which plays an essential role in the emotional management and processing. The SLF is an important mediator of white matter connectivity within the neural circuit of frontal, parietal and temporal lobes, and it is also associated with mood regulation and cognitive function6. The corpus callosum plays an essential role in relaying sensory, motor, and cognitive information across cerebral hemispheres7. Our study found lower NQA and GFA in cingulum of MDD_S, and lower GFA in SLF of MDD_N. Lower NQA and GFA in cingulum might due to the abnormal white matter completeness and lead to the dysfunction of emotional processing8; lower GFA in SLF also might due to the abnormal white matter completeness, such as demyelination or the change of the intensity of fiber in SLF which might cause MDD9. Lower NQA and GFA in the corpus callosum might contribute to the functional alterations in the inter-hemispheric system of emotional regulation1. Higher ISO in the frontal lobe could be explained because of the pathological processes of reducing the neuronal size and glial cell density9, 10.Conclusion
In this study, we used GQI with voxel-based statistical analysis to observe the brain structural changes among MDD_N, MDD_S, and HC. We found the significant differences in the corpus callosum, SLF, cingulum, and frontal lobe of MDD_N or MDD_S compared to HC, and in the corpus callosum and frontal lobe between MDD_N and MDD_S. These findings not only give us an opportunity to classify the MDD patients if they have potential self-harm behavior but also may serve as an imaging mark for clinical diagnosis.Acknowledgements
This study was supported in part by the research programs NSC102-2314-B-182-068-MY3 and MOST105-2314-B-182-028, which were sponsored by the Ministry of Science and Technology, Taipei, Taiwan.References
1. Kieseppä T, Eerola M, Mäntylä R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010; 120(1-3): 240-244.
2. Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013; 38(1): 49-56.
3. Tymofiyeva O, Connolly CG, Ho TC, et al. DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J Affect Disord. 2016; 207: 18-25.
4. Bracht T, Linden D, Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J Affect Disord. 2015; 187: 45-53.
5. Srivastava S, Bhatia MS, Bhargava SK, et al. A Diffusion Tensor Imaging Study Using a Voxel-Based Analysis, Region-of-Interest Method to Analyze White Matter Abnormalities in First-Episode, Treatment-Naïve Major Depressive Disorder. J Neuropsychiatry Clin Neurosci. 2016; 28(2): 131-137.
6. Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005; 15(6): 854-869.
7. de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985; 44(6): 578-591.
8. Beauregard M, Paquette V, Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. NeuroReport. 2006; 17(8): 843-846.
9. Chen VC, Shen CY, Liang SH, et al. Assessment of abnormal brain structures and networks in major depressive disorder using morphometric and connectome analyses. J Affect Disord. 2016; 205: 103-111.
10. Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999; 45(9): 1085-1098.
Figures