4226
Utility of MR-Spectroscopy in Early Drug Discovery: Characterization of Psychiatric Dysfunctions & Psycho-active Drug Effects1Singapore Bioimaging Consortium, Singapore, Singapore, 2Neuroscience Therapeutic Area, Janssen Pharmaceuticals R&D, Belgium
Synopsis
MR-Spectroscopy offers a unique potential to characterize underlying neuronal mechanisms of psychiatric dysfunctions and the effects of psychoactive drugs, at a fundamental neuro-metabolite level in-vivo. We studied two preclinical disease models: the chronic mild unpredictable stress (CMUS) model, a putative model for depression, and a sub-chronic memantine (NMDA antagonist) model, a putative model for psychosis using single voxel spectroscopy (SVS). Our results represents metabolic fingerprinting of dysfunctions utilising live metabolic flux profiling; documenting neuro-metabolic effects of novel psycho-active drugs, presenting novel insights in-vivo. Our results and unique approach, exemplifies the potential value of SVS in early stage drug discovery and its potential translation to clinical research.
Introduction:
MR-Spectroscopy offers a unique potential to characterize underlying neuronal mechanisms of psychiatric dysfunctions and the effects of psychoactive drugs, at a fundamental neuro-metabolite level in-vivo. We studied two preclinical disease models: the chronic mild unpredictable stress (CMUS) model, a putative model for depression, and a sub-chronic memantine (NMDA antagonist) model, a putative model for psychosis using single voxel spectroscopy (SVS). We have systematically established the basis for this investigation through a series of behavioural (hyperlocomotion, pre-pulse inhibition, sucrose intake) as well as imaging (phMRI, rs-fMRI, DKI, preliminary SVS) findings in the past [1(a-f)]. Here we extend the line of investigation through a comprehensive experimental design and robust MRS protocol, to further advance our scientific understanding of neuronal changes linked to animal models of disease. Neuro-metabolic effects of a series of novel psycho-active compounds, with anti-psychotic potential are also documented, exemplifying the value of this in-vivo imaging readout, in early stage drug discovery research.
Methods & Materials:
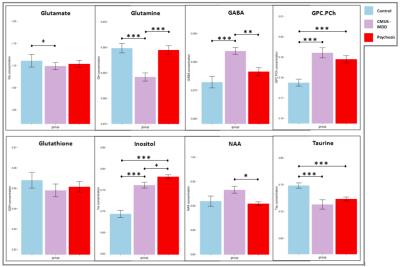
Every cohort had ‘n’ of 6 Wistar (male) rats. The CMUS cohorts were subjected to a robust 28 days long, chronic mild unpredictable stress behavioural protocol [1-f], while the memantine cohorts were subjected to 5 days of sub-chronic IP injection of the NMDA antagonist memantine, at a dose of 20 mg/kg [1-a]. A set of four SVS spectra were acquired in both disease models, prior to any drug challenge and the weighted average of the metabolites are reporting [Figure 1]. For psycho-active drug characterization, rats were initially challenged with either an mGlu2 PAM (JNJ-42153605, 10 mg/kg, PO or BINA, 32 mg/kg, IP), an mGlu2/3 agonist (LY404039, 10 mg/kg, PO), vehicle or saline, during subject preparation for imaging. Subsequently subjects were loaded in the scanner, with volume transmit and phased array receive coil set-up. This pre-treatment cum set-up duration was 90 mins. Upon voxel positioning and shimming procedures, the challenge drug (acute bolus of memantine 20 mg/kg, IP or saline) was administered and continuously temporal response - metabolite flux was documented (for a duration of 60 mins). Data acquired was grouped into 3 equally weighted bins, 0-20, 20-40 & 40-60 mins.Data acquisition:
An ultra high field 9.4T MRI (Bruker-Biospec) scanner was employed for data collection [TR 4000; TE 13ms; NA 128; 2.5×4×4 mm3 voxel positioned on the hippocampus; TA 8.40 mins/spectrum]. In order to correct for eddy current compensation and scaling, a water unsuppressed spectrum was documented for every animal. Significant efforts were made during voxel positioning and shimming to achieve a consistent effective FWHM line-width of 8.4 to 11.8 HZ in every animal/spectrum acquired. LC model was used for quantitation of the generated spectrums. Statistical Analysis: Metabolite concentrations relative to total creatine were fitted into a linear model using Cramér–Rao lower bound as weighting factor on the residuals to minimize the influence of poorly fitted metabolites. Comparisons between groups was carried out using parametric contrast analysis. Statistical significance corresponded to p<0.05.Results & observations:
CMUS led to significant changes in the neuronal metabolism predominantly in the following metabolites: glutamate, GABA, inositol, taurine and total visible choline (phosphocholine and glycerophosphocholine). Marginal significance was observed in glutamate levels. Similarly sub-chronic memantine treatment led to significant changes in the inositol, taurine and total visible choline levels. Our findings in glutamine (& glutamate) levels relative to the total creatine prove consistent with the biogenic amine depletion hypothesis of depression. Changes in total creatine and inositol potentially reflects membrane metabolism changes and inflammation, respectively [Figure 1]. These findings are also consistent with our recent investigation in a chronic social defeat model [2]. Amongst the 3 bins of data, looking at the last bin (40th to 60th min): pre-treatment of mGlu2 PAMs JNJ-42153605 and BINA inhibited the memantine induced effects on glutamate & glutamine; whereas the mGlu2/3 agonist LY404039 did not, indicating that the mGlu2/3 agonist has distinct effects compared to the PAMs, which needs further investigation. Interestingly, a further pronounced significant effect of JNJ-42153605 pre-treatment on GABA was observed, compared to other cohorts [Figure 2]. Our results represents metabolic fingerprinting of dysfunctions utilising live metabolic flux profiling; documenting neuro-metabolic effects of novel psycho-active drugs, presenting novel insights in-vivo. Our results and unique approach, exemplifies the potential value of SVS in early stage drug discovery and its potential translation to clinical research.Acknowledgements
JCO Development Platform Grant, A*Star, Singapore