4225
The acute pharmacological MRI response to a citalopram challenge is modulated by earlier selective serotonin reuptake inhibitor exposure in an age dependent manner1Department of Radiology, Academic Medical Center Amsterdam, Amsterdam, Netherlands, 2Swammerdam Institute for Life Sciences, Center for Neurosciences, University of Amsterdam, Amsterdam, Netherlands, 3Sunnybrook Research Institute, University of Toronto, Toronto, Canada
Synopsis
Preclinical studies have shown that selective serotonin reuptake inhibitor (SSRI) treatment, when applied to the developing brain, is associated with long-term changes in the adult serotonergic system. Using pharmacological MRI (phMRI), we here investigated whether SSRIs can also induce such age-dependent changes in the human serotonergic system. We found that the phMRI response to citalopram was decreased in the amygdala only in adult female subjects who had been first exposed to SSRIs early in life, whereas a blunted response was found in subjects first exposed at a later age.
PURPOSE
Selective serotonin reuptake inhibitors (SSRIs), such as citalopram, are the main pharmacological treatment for major depressive disorder (MDD), not only in adults, but also often in children. Although safety of SSRIs in adults is well established1, limited data is available on the long-term effects of these drugs, particularly on the developing serotonergic system2. Moreover, preclinical studies have shown that SSRIs have different effects when administered during brain development or during adulthood3. Particularly areas in the limbic system - cingulate cortex, amygdala, thalamus - are of interest, as serotonin receptor availability in these regions is affected by MDD4. In humans, however, it is still unknown whether SSRI effects on the serotonergic system also depend on age of first exposure. Therefore, we measured whether the pharmacological MRI (phMRI) response to a citalopram challenge is modulated by age of first SSRI exposure. PhMRI is a non-invasive imaging technique that can be used as a proxy for serotonin function5 by measuring changes in cerebral blood flow (CBF) induced by the SSRI citalopram.METHODS
PhMRI with a citalopram challenge was performed in 43 female subjects with (prior) MDD. Subjects were stratified into 3 groups: unexposed (NO, never received SSRI treatment), early exposed (EARLY, received SSRIs < 23 years) and late exposed (LATE, received SSRIs > 23 years) subjects (Table 1 and Figure 1a). Those participants currently on anti-depressants were medication-free for at least three weeks. For CBF assessments, we used pseudo continuous arterial spin labeling (pCASL) acquired on a 3.0T Ingenia (Philips, Best, the Netherlands) using 16-channel receive-only head coil with the following parameters: 2D EPI readout; TR/TE = 4000/14 ms; post-label delay = 1525 ms; label duration = 1650 ms; FOV = 240x240 mm; 17 7 mm slices, voxel size = 3x3x7 mm; number of dynamics = 183 (Figure 1b). In addition, M0 and 3D T1 scans were obtained. The citalopram challenge was injected i.v. (7.5 mg) 5 minutes after start of the phMRI scan (Figure 1b). ASL post-processing was performed with the ExploreASL toolbox6, to obtain pre- and post-citalopram cerebral blood flow (CBF) images (Figure 1b). In short, T1w images were segmented into gray matter (pGM) and white matter (pWM) probability maps. Motion was estimated and motion spikes were excluded. Perfusion-weighted images were rigid-body registered to the pGM images. CBF was quantified using a single compartment model7. The pGM and pWM maps were spatially normalized using DARTEL8, and all transformations were combined into a single interpolation to transform the CBF maps to MNI space. Change in CBF (ΔCBF) was calculated as the difference in CBF pre- and post-citalopram. Statistical significance was assessed by comparing ΔCBF values between the groups using analysis of variance (ANOVA) and analysis of covariance (ANCOVA) when covariates are included for four ROIs: thalamus, amygdala (Figure 2a), anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC).RESULTS
ANCOVA showed a significant interaction between group and time-point (F=3.625, p=0.036) after correction for the covariates age9 and current depressive score10,11. Post-hoc tests showed that the mean ΔCBF in the amygdala differed between the NO and LATE subjects (F=5.728 p=0.026) and between the EARLY and LATE subjects (F=8.575 p=0.007), but not between the NO and EARLY subjects (F=0.647, p=0.429) (Figure 2b). No significant effects were found for the other ROIs (Figure 2b).DISCUSSION
Adult female subjects who were first exposed early in life showed a decrease in amygdala CBF after the citalopram challenge, whereas subjects first exposed later in life showed a blunted response, suggesting that the effects of SSRIs depend on age of first exposure. One possible explanation could be that in the LATE subjects, but not the EARLY subjects, a lasting desensitization of serotonin auto-receptors may have occurred12, resulting in decreased responsiveness to an acute serotonin challenge. Because the phMRI response in the EARLY group was comparable to the NO group, the auto-receptors in the EARLY group may not have undergone desensitization, presumably due to a higher degree of neuroplasticity in the developing brain. Another possibility could be that different neurobiological mechanisms underly the depressive symptoms in patients developing MDD later in life, i.e. the LATE group, compared to EARLY-onset depression13.Acknowledgements
No acknowledgement found.References
1. Racagni G, Popoli M (2008): Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci 10:385–400.
2. Hetrick S, Merry S, McKenzie J, Sindahl P, Proctor M (2007): Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane database Syst Rev:CD004851.
3. Klomp A, Tremoleda JL, Wylezinska M, Nederveen AJ, Feenstra M, Gsell W, Reneman L (2012): Lasting effects of chronic fluoxetine treatment on the late developing rat brain: Age-dependent changes in the serotonergic neurotransmitter system assessed by pharmacological MRI. Neuroimage 59:218–226.
4. Kambeitz JP, Howes OD (2015): The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J Affect Disord 186:358–366.
5. Chen Y, Wan HI, O’Reardon JP, Wang DJJ, Wang Z, Korczykowski M, Detre JA (2011): Quantification of Cerebral Blood Flow as Biomarker of Drug Effect: Arterial Spin Labeling phMRI After a Single Dose of Oral Citalopram. Clin Pharmacol Ther 89:251–258.
6. Mutsaerts HJ, Thomas DL, Petr J, et al. (2016). Addressing multi-centre image registration of 3T arterial spin labeling images from the GENetic Frontotemporal dementia Initiative (GENFI). In: International Society for Magnetic Resonance in Medicine.
7. Alsop DC, Detre JA, Golay X, et al. (2014). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med: 73(1):102-116.
8. Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage: 38(1):95-113.
9. Martin a J, Friston KJ, Colebatch JG, Frackowiak RS (1991): Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689.
10. Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C (1986): The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res 18:65–87.
11. Drevets WC, Bogers W, Raichle ME (2002): Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol 12:527–544.
12. Sekar S, Verhoye M, Audekerke J Van, Vanhoutte G, Lowe AS, Blamire AM, et al (2011). Neuroadaptive responses to citalopram in rats using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 213: 521–31.
13. Naismith SL, Norrie LM, Mowszowski L, Hickie IB (2012): The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol 98:99–143.
Figures
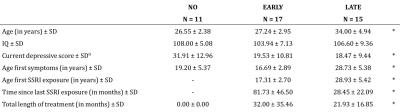
Table 1 – Patient characteristics
* ANOVA p < .05
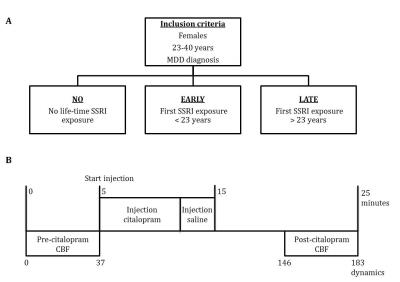
Figure 1 – Designs
a) Study groups
b) Time-line phMRI scan
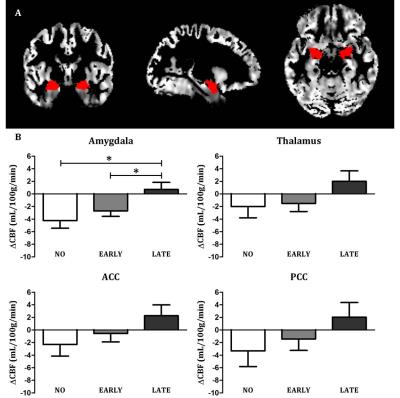
Figure 2 – Results
a) Gray matter CBF image of a representative subject with the bilateral amygdala mask overlaid
b) Mean change in CBF (ΔCBF) for the four ROIs for each group (amygdala, thalamus, anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC), adjusted for age and current depressive score. * = Post-hoc ANCOVA p < .05