4220
Proton magnetic resonance spectroscopy study of amygdala, anterior cingulate cortex and thalamus in pediatric post-traumatic stress disorder patients1Department of Radiology, West China Hospital of Sichuan University, Chengdu, People's Republic of China, 2West China Hospital of Sichuan University, People's Republic of China
Synopsis
PTSD is associated with a variety of structural and functional brain abnormalities, but the molecular pathophysiological mechanisms are unknown. 25 pediatric PTSD and 24 matched healthy control subjects underwent single voxel 1H-MRS. Right amygdala NAA was significantly increased in pediatric PTSD subjects than in controls, and the other metabolites did not differ significantly between the groups. We hypothesis that long-term excessive activation in amygdala after traumatic events may lead to increase density and activity of the neurons in pediatric PTSD patients with increased NAA concentration, which may be an adaptive response to traumatic stimulation in the human brain. Our findings add the neurochemical abnormality evidence in pediatric PTSD.
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating disorder in which an acute fear response to a traumatic event just like earthquake does not abate. Instead, patients continue to experience a number of emotional and behavioral dysregulation symptoms, including intrusive recollections of the trauma, hyperarousal and hypervigilance, emotional numbing, and avoidance of trauma reminders. Although PTSD is associated with a variety of structural and functional brain changes, the molecular pathophysiological mechanisms underlying these macroscopic alterations are unknown. This study was designed to evaluate the neurochemical changes in bilateral amygdala, anterior cingulate cortex (ACC) and bilateral thalamus of pediatric PTSD patients after an earthquake.Method
Twenty-five pediatric PTSD diagnosed by DSM-IV and twenty-four matched healthy control subjects underwent single voxel 1H-MRS at 3 Tesla MR scanner. The severity of symptoms was assessed by the PTSD Checklist-Civilian Version (PCL-C) (1) and Clinician Administered PTSD Scale for Children and Adolescents (CAPS-CA) (2). Metabolites including N-acetylaspartate (NAA), choline (Cho), glutamate+glutamine (Glx), myo-inositol (mI) and creatine (Cr) were measured in the bilateral amygdala, ACC and bilateral thalamus (see Figure 1), using Point-Resolved Echo Spectroscopy Sequence (PRESS). The differences of each metabolite between patients and controls were evaluated. Correlation between the concentration of metabolites and PCL-C scores and CAPS-CA scores were also studied.Result
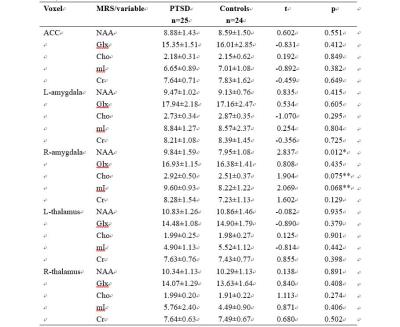
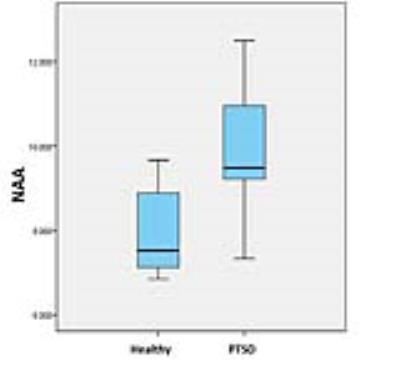
The two groups were matched on age and gender. Pediatric PTSD patients scored significantly higher than controls on PCL-C scores. Patients’ CAPS-AC scores reflect PTSD symptoms in the moderate-to-severe range (Table 1). Right amygdala NAA was significantly higher in pediatric PTSD subjects than in controls (uncorrected P=0.012), and the other metabolites did not differ significantly between the groups in ACC, bilateral amygdala and bilateral thalamus (Table 2). Right amygdala NAA concentration was not significantly associated with severity of PTSD symptoms.Discussion
With numerous studies about PTSD previously, the neurocircuitry models of PTSD termed ‘fear circuitry’ were proposed: key components involves amygdala, hippocampus, ACC, prefrontal cortex, thalamus and insula (3-6). Unlike other structures, MRS in amygdala has rarely been studied due to its small volume. However, we improve spectral quality of amygdala by applying space saturation technique without additional scanning time. As we all know, NAA represents the marker of neuron integrity. The main finding of our study was that the right amygdala NAA concentration of pediatric PTSD subjects was significantly higher than that of healthy controls (Figure 2). Contrary to our finding, many other studies including research in animals reported declined NAA concentration or NAA/Cr (7-9). However, it is worthwhile to note that the enrolled samples in the present study were children and adolescents. Furthermore, one study has showed that the NAA concentration of normal children will be increased with gradual maturation of the central nervous system, reaching the peak at 10 years old (10). On the other hand, the NAA concentration will gradually reduced due to the degeneration of neurons in middle age (11). What above mentioned may explain the inconsistent results. We hypothesis that long-term excessive activation in amygdala after traumatic events may lead to increase density and activity of the neurons in pediatric PTSD patients with increased NAA concentration, which may be an adaptive response to traumatic stimulation in the human brain.Conclusion
In summary, 1H-MRS NAA concentration in the right amygdala, which was thought to be the most implicated brain structure in the pathophysiology of PTSD, was significantly increased in pediatric PTSD patients versus matched healthy subjects though this may be due to the small sample. This finding may be consistent with evidence of amygdala exaggerated acquisition of fear associations and expression of fear responses of PTSD. No group differences in other metabolites were found in the bilateral amygdala, ACC and bilateral thalamus. Our findings add the neurochemical abnormality evidence in pediatric PTSD.Acknowledgements
This study was supported by the National Natural Science Foundation (Grant No.81030027) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1272) of China.References
1. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM: The PTSD Checklist: Reliability, validity, and diagnostic utility. in Annual meeting of the International Society for Traumatic Stress Studies. San Antonio (USA)1993.
2. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of traumatic stress. 1995;8:75-90.
3. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164:1476-1488.
4. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:169-191.
5. Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry. 2006;60:376-382.
6. Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:56-72.
7. Shu XJ, Xue L, Liu W, Chen FY, Zhu C, Sun XH, Wang XP, Liu ZC, Zhao H. More vulnerability of left than right hippocampal damage in right-handed patients with post-traumatic stress disorder. Psychiatry research. 2013;212:237-244.
8. Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, Kornak J, Marmar CR, Weiner MW. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry research. 2008;162:147-157.
9. Siegmund A, Kaltwasser SF, Holsboer F, Czisch M, Wotjak CT. Hippocampal N-acetylaspartate levels before trauma predict the development of long-lasting posttraumatic stress disorder-like symptoms in mice. Biological psychiatry. 2009;65:258-262.
10. Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. Journal of magnetic resonance imaging : JMRI. 2002;15:137-143.
11. Raininko R, Mattsson P. Metabolite concentrations in supraventricular white matter from teenage to early old age: A short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta radiologica (Stockholm, Sweden : 1987). 2010;51:309-315.
Figures

Table 1. Clinical and demographic data. Note: Mean ± SD. a Posttraumatic Stress Disorder Checklist; b Clinician Administered PTSD Scale.