4219
Brain Cortical Thickness in Adolescents from Multiplex Alcohol Dependence Families1Centre for Addiction Medicine and Department of Psychiatry, National Institute of Mental Health & Neuro Sciences (NIMHANS), Bangalore, India, 2Cognitive Neuroscience Centre and Department of Neuroimaging and Interventional Radiology (NIIR), National Institute of Mental Health & Neuro Sciences (NIMHANS), Bangalore, India, 3Translational Psychiatry Laboratory and Department of Psychiatry, National Institute of Mental Health & Neuro Sciences (NIMHANS), Bangalore, India
Synopsis
Adolescents with high
- Introduction
The transition from childhood to adolescence represent a key developmental period with heightened risk for drug experimentation and onset of problem addictive behaviors 1, 2. Furthermore, adolescents with high familial alcoholism have further elevated-risk for developing alcohol-use-disorders (AUDs) compared to their peers without such family-history (FH) 3, 4. These High-Risk (HR) offspring differ from their typically developing Low-Risk (LR) control peers on a variety of psychological and neurobiological precursors of AUDs 5, 6. These include premorbid subcortical 7-10 and cerebellar 11 brain volumetric alterations. They also differ in brain activity during executive-functioning, reward, and emotion-processing tasks 5. However, the changes in the maturation of the Cortical Thickness (CTh) measures during the adolescence in HR offspring and their relationship with the externalizing behaviors have never been examined. - Methods
2.1. Participants
Eighty alcohol-naïve male HR-offspring of treatment-seeking AUD patients were recruited from a tertiary-care neuropsychiatry hospital. The affected father was required to have established AUD (DSM-IV criteria) before the age of 25 (early-onset) and have at least two further affected first-degree relatives (FDR) with AUD. Seventy alcohol-naïve typically-developing LR-control subjects (without alcohol/ other substance-dependence FH in FDR), matched on age-, education-, sex-, handedness- and socioeconomic-status to the HR group were recruited for comparisons.
2.2. Clinical assessments
All subjects were assessed on SSAGA-II 12 to compute an externalizing symptoms score (ESS) and to rule out any syndromal psychiatric and substance-use disorders. FIGS 13 was used to document alcoholism FH and to screen for other psychiatric disorders. The HR offspring with the mother having diagnosable AUDs or alcohol use during the index pregnancy were excluded, to rule out fetal-alcohol effects.
2.3. MRI acquisition and Processing
The MRI scans of the whole-brain were obtained with a Siemens 3T Skyra-MRI (Erlangen, Germany) using a 32-channel head-coil. A T1-weighted, three-dimensional, high-resolution MPRAGE was performed (TR=1900ms, TE=2.43, TI=900ms, FOV=240*240mm2, slice thickness=0.9mm with no gap) yielding 192 sagittal-slices with voxel size of 1*1*1mm3. All individual images were initially screened for motion-artifacts and gross structural abnormalities. Surface-based CTh reconstruction from T1-weighted MRIs were performed using FreeSurfer Software (v5.3.0, https://surfer.nmr.mgh.harvard.edu/) 14, 15. The CTh maps were smoothed on the surface using a 15mm full-width half-maximum Gaussian kernel. Analyses were conducted using a GLM, controlling for age (linear and quadratic-effects), and head-size (eTIV) to examine the differences in CTh developmental trajectories and effect of ESS. The resulting CTh maps were corrected for multiple-comparisons using a Monte-Carlo-Null-Z simulation, performing 10,000 iterations with a final cluster-wise p <0.05.
Results
2.1. Participant Characteristics
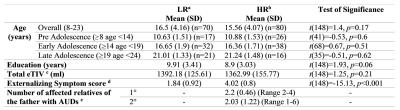
All 150 participants were right-handed, male, substance-naïve, and between 8-25y of age. The groups did not differ in mean age, education, and eTIV. The HR group exhibited significantly greater summative counts of externalizing symptoms and a high family loading of AUDs.
2.2. Comparison of CTh measures
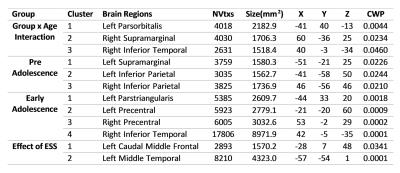
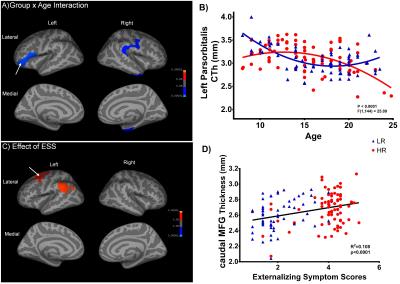
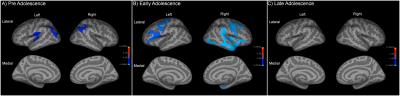
There was no significant group-by-age interaction when only considering the linear growth models. For the quadratic age models, however, there was significantly less age-related thinning observed in HR group in the clusters over left-parsorbitalis, and right ITG, supramarginal and insula regions than LR group. In the background of significant group-by-age interaction, the whole sample was split into three subgroups of pre-adolescents (8-13y), early-adolescents (14-18y) and late-adolescents (19-24y) in order to examine the between-group main-effects within these developmental-spans. In the pre-adolescents group, HR subjects had lesser thinning than LR in bilateral inferior-parietal, left supramarginal regions. In the early-adolescents group, HR subjects had wide spread areas with lesser thinning in comparison to LR controls involving left pars-triangularis, bilateral precentral, and right ITG regions. Interestingly, there were no significant between-group main-effects for the late-adolescent groups.
2.3. Correlation with externalizing symptoms
The entire cortex-wise regression analysis revealed a positive relationship between the ESS and CTh of the left caudal MFG and MTG.
Discussion
The current study involved characterization of CTh maturation in a large and well-characterized sample of substance-naïve male-subjects with/without AUD-FH. Our findings indicate a lag in the CTh developmental trajectories of HR-offspring when compared to neurotypical LR controls. In HR group, the CTh continues to peak into early-adolescence period followed by a decline, possibly suggesting delayed gray-matter pruning for age16. Moreover, in-accordance with previous literature17, cortical thinning was associated with better behavioral control across the groups, as indicated by the correlational analysis with ESS. Interestingly, their CTh maturation starts to catch-up with the LR group by late adolescence. Taken together, these results suggest wide-spread delays in cortical maturation in HR subjects, which may ultimately contribute to their addiction vulnerability. More importantly, these effects reduce with age by late-adolescence in the absence of substance-misuse.Acknowledgements
This study was supported by the Centre for Addiction Medicine, Department of Psychiatry, National Institute of Mental Health & Neuro Sciences (NIMHANS), Bangalore, India. Author BH is supported by the Wellcome Trust/DBT India Alliance. None of the authors have any financial disclosure to make or have any conflict of interest.References
1. Chambers, R.A., J.R. Taylor, and M.N. Potenza, Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry, 2003. 160(6): p. 1041-52.
2. Petit, G., et al., Why is adolescence a key period of alcohol initiation and who is prone to develop long-term problem use?: A review of current available data. Socioaffect Neurosci Psychol, 2013. 3: p. 21890.
3. Cloninger, C.R., et al., Inheritance of risk to develop alcoholism. NIDA Res Monogr, 1986. 66: p. 86-96.
4. Schuckit, M.A., Genetics and the risk for alcoholism. JAMA, 1985. 254(18): p. 2614-7.
5. Cservenka, A., Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend, 2016. 158: p. 8-21.
6. Hill, S.Y. and J. O'Brien, Psychological and Neurobiological Precursors of Alcohol Use Disorders in High Risk Youth. Curr Addict Rep, 2015. 2(2): p. 104-113.
7. Benegal, V., et al., Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol, 2007. 12(1): p. 122-32.
8. Cservenka, A., et al., Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J Stud Alcohol Drugs, 2015. 76(1): p. 47-56.
9. Hill, S.Y., et al., Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry, 2001. 49(11): p. 894-905.
10. Sjoerds, Z., et al., Family history of alcohol dependence and gray matter abnormalities in non-alcoholic adults. World J Biol Psychiatry, 2013. 14(8): p. 565-73.
11. Hill, S.Y., et al., Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry, 2007. 61(1): p. 41-7.
12. Bucholz, K.K., et al., A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol, 1994. 55(2): p. 149-58.
13. Maxwell, M., Family Interview for Genetic Studies (FIGS): a manual for FIGS, clinical neurogenetics branch, intramural research program. National Institute of Mental Health, Bethesda, MD, 1992.
14. Dale, A.M., B. Fischl, and M.I. Sereno, Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 1999. 9(2): p. 179-94.
15. Fischl, B., M.I. Sereno, and A.M. Dale, Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage, 1999. 9(2): p. 195-207.
16. Paus, T., Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci, 2005. 9(2): p. 60-8.
17. Squeglia, L.M., et al., Early adolescent cortical thinning is related to better neuropsychological performance. J Int Neuropsychol Soc, 2013. 19(9): p. 962-70.
Figures