4218
Accumulation of Prefrontal Lactate Levels in Chronic Schizophrenia1Center for Biomedical Imaging (CIBM), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Unit for Research in Schizophrenia, Center for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 3Service of General Psychiatry, Department of Psychiatry, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 4Laboratory of Functional and Metabolic Imaging, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 5Departments of Radiology, University of Lausanne and Geneva, Lausanne and Geneva, Switzerland
Synopsis
Mitochondrial dysfunction including altered brain energy metabolism has been implicated in the pathophysiology of schizophrenia. The aim of this study was to investigate prefrontal lactate(Lac) levels in patients with chronic schizophrenia. An increase of [LacmPFC] was observed in patients with chronic schizophrenia relative to healthy controls. This may be associated with glucose metabolism impairment and mitochondrial dysfunction resulting from oxidative stress. Indeed, oxidative damages can impair mitochondrial oxidative phosphorylation and enzyme activities of pyruvate dehydrogenase, leading to Lac production. Therefore, this study provides in vivo evidence supporting that oxidative stress and mitochondrial dysfunction may be involved in schizophrenia.
Introduction
Mitochondrial dysfunction including altered brain energy metabolism has been implicated in the pathophysiology of schizophrenia (SZ). Reduced kinetics of creatine kinase and phosphodiester to ATP ratio has been revealed in patients with chronic schizophrenia1. Moreover, elevated lactate (Lac) levels have been found in the cerebrospinal fluid2 and postmortem brains of patients3 with chronic schizophrenia. Interestingly, an accumulation of Lac was also observed in a study of glutathione-deficient schizophrenia mouse model4. However, in vivo brain Lac levels in patients with schizophrenia have not yet been reported. Therefore, the aim of this study was to investigate prefrontal Lac levels in patients with chronic schizophrenia using 1H MRS in comparison with matched controls.Methods
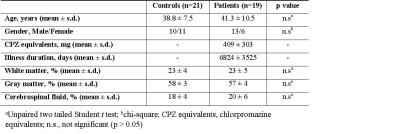
19 patients with chronic schizophrenia were recruited from the outpatient clinic of the department of psychiatry, Lausanne University Hospital, Switzerland, and met criteria DSM-IV for schizophrenia or schizoaffective disorder5 and 21 control subjects were recruited from similar geographic and socio-demographic areas through advertisement and were assessed by the Diagnostic Interview for Genetic Studies6. All subjects gave informed consent prior to the study.
All MRS measurements were performed on a 3T Trio MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a TEM volume coil. B0 field inhomogeneity was optimized using first- and second-order shimming with FAST(EST)MAP. 1H MR spectra were obtained in the voxel located in medial prefrontal cortex (mPFC) using the SPECIAL7 localization sequence (TE/TR=6/4000ms, VOI=20×20×25mm3, 148 averages). Metabolite concentrations were quantified with LCModel8 using unsuppressed water MR spectra as an internal reference. Tissue composition within the MRS voxel was evaluated based on the segmentation of 3D MPRAGE images. Fractions of white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF) between patients and controls were compared using two-tailed t-test. The effect of disease, age, gender, SNR and linewidth on lactate concentration was investigated using generalized linear model. All values were reported as mean ± sd. Spearman correlation was used to investigate the correlation between [LacmPFC] and medication dose.
Results
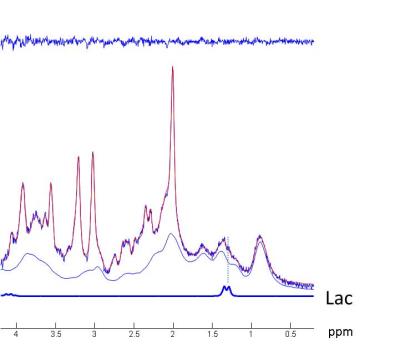
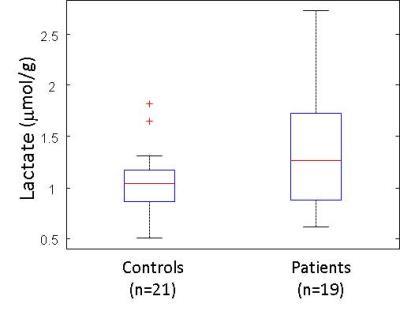
Between patients and controls no statistical differences was observed in age and gender (Table 1). 1H MR spectra in the mPFC of one patient and the corresponding spectral fits are shown in Figure 1. SNR (NAA peak height) and linewidth were 95 ± 10, 0.034 ± 0.007ppm for controls and 90 ± 7, 0.038 ± 0.007ppm for patients, respectively. No significant differences (paired t-test) in spectral quality (i.e. SNR and linewidth) between groups were observed, indicating comparable spectral quality between groups. [LacmPFC] was quantified with a CRLB of 11 ± 4%. A 32% increase (p=0.048) of [LacmPFC] was observed in patients (1.39 ± 0.65mmol/g) with chronic schizophrenia relative to matched healthy controls (1.05 ± 0.37mmol/g) (Figure 2). This result still remains significant by including age, gender, linewidth and SNR as covariates (p<0.05). There is also no correlation observed between [LacmPFC] and CPZ equivalents.Discussion
[LacmPFC] of controls (1.05 ± 0.37mmol/g) and CRLBs (%) are in excellent agreement with reported values in the frontal lobe9, which is higher than the levels in occipital cortex and posterior cingulate. High quality spectra without lipid contamination obtained at 3T allows the measurement of lactate as shown in Fig.2, i.e. a distinct peak of the right side doublet of lactate at 1.3ppm. The increase of [LacmPFC] in chronic schizophrenia is in line with observations of increased Lac in the CSF of patients with schizophrenia2, which may be associated with glucose metabolism impairment and mitochondrial dysfunction. In addition, the elevation of Lac may also be the result of oxidative stress. It has been reported that oxidative damages can impair mitochondrial oxidative phosphorylation and enzyme activities of pyruvate dehydrogenase, leading to lactate production2. Indeed, the late accumulation of lactate was observed in the glutathione-deficient schizophrenia mouse model4 which has increased sensitivity to oxidative stress10. Recently, a significant reduction in the ratio of redox pair of Nicotinamide adenine dinucleotide, i.e. oxidized form (NAD+) and reduced form (NADH), was reported in chronic SZ patients11, implying the presence of oxidative imbalance and potentially oxidative stress in SZ. In glycolysis, lactate dehydrogenase converts pyruvate to lactate or back by recycling NADH to NAD+ or vice versa. Therefore, to counterbalance NAD+/NADH in the presence of reduction of NAD+/NADH, the conversion of pyruvate to lactate may be driven, resulting in accumulation of lactate. Therefore, we conclude that this study provides in vivo evidence supporting that oxidative stress and mitochondrial dysfunction may be involved in the pathophysiology of schizophrenia.Acknowledgements
Supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenards and Jeantet Foundations, and the Swiss Bridge Foundation, the Swiss National Science Foundation (No320030_122419), and the National Center of Competence in Research (NCCR) ‘SYNAPSY—The Synaptic Bases of Mental Diseases’ (No. 51AU40_125759). We thank the ‘Loterie Romande’, Stanley Thomas Johnson, Damm-Etienne, Avina and Alamaya Foundations.References
1. Du F et al., JAMA Psychiatry, 2014;71(1) :19-27.
2. Prabakaran S et al., Mol Psychiatry, 2004 ;(9):684 –97.
3. Regenold W T et al., Biol Psychiatry, 2009; 65(6): 489–494.
4. Das Neves Duarte JM et al, Biol Psychiatry, 2012 ; 71:1006-14.
5. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. DSM-IV-TR. American Psychiatric Pub, Arlington, 2000;VA22209, USA.
6. Preisig M et al., Eur Arch Psychiatry Clin Neurosci, 1999; 249: 174–179.
7. Mekle R et al., Magn Reson Med., 2009; 61:1279-85.
8. Provencher SW, Magn Reson Med., 1993; 30:672-9.
9. Emir U E et al., NMR Biomed ,2012; 25(1): 152–160.
10. Yang Y et al., J Biol Chem, 2002 ;(277): 49446-49452.
11. Kim SY et al., Schizophr Bull., 2016 [Epub ahead of print].
Figures