4217
Aberrant fronto-limbic effective connectivity during repeated fearful face stimuli in body dysmorphic disorder and anorexia nervosa1Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, United States, 2AU MRI Research Center, Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 3Imaging Genetics Center, Department of Neurology, University of Southern California, Los Angeles, CA, United States, 4Oxley College of Health Sciences, University of Tulsa, Tulsa, OK, United States, 5Laureate Institute for Brain Research, University of Tulsa, Tulsa, OK, United States, 6Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, United States
Synopsis
Anorexia nervosa (AN) and body dysmorphic disorder (BDD) share distorted perception of appearance, anxiety, and depression, yet their common and distinguishing neural phenotypes of emotion processing remain unknown. To address this, we studied fronto-limbic connectivity using functional MRI data obtained while participants (N=94) viewed fearful faces and rated their own subjectively experienced fearfulness. Healthy controls exhibited, as predicted, significant bidirectional medial prefrontal (mPFC)-amygdala connectivity, which increased across blocks. However, BDD participants exhibited significant mPFC-to-amygdala but not amygdala-to-mPFC connectivity (indicating limbic hypo-responsiveness), while AN exhibited no significant prefrontal-amygdala connectivity. This study suggests distinct, aberrant fronto-limbic modulatory connectivity in AN and BDD.
Introduction
Body dysmorphic disorder (BDD) and anorexia nervosa (AN) are psychiatric disorders of relatively high prevalence in the general society (BDD: ~2%1; AN: ~1% in females2). BDD is typically characterized by focus on perceived flaws of the face and head3, while AN is distinguished by extraordinary fear of normative weight and restrictive eating. These disorders are differently classified4 despite common characteristics of distorted visual perception. Moreover, similar abnormal brain activation patterns in visual systems were previously found5. To date, common and distinguishing neural phenotypes of BDD and AN remain under-explored. Emergence of anxiety precedes wright loss and body image disturbance in development in AN6, which suggests that limbic system dysfunction might possibly contribute to AN. In BDD, however, individuals perceive their faces as aversive, yet do not exhibit hyperactivity in limbic regions7. Motivated by these observations, we examined brain connectivity during repeated exposure to fearful faces, to probe fronto-limbic modulation of emotion-regulating circuitry8. We studied and compared directional connectivity between bilateral medial-prefrontal cortex (mPFC), rostral anterior-cingulate cortex (rACC) and amygdala8,9 in BDD, AN and healthy controls. We hypothesized that, with repeated exposure to fearful faces: (i) controls would exhibit significant bidirectional fronto-limbic connectivity; (ii) BDD participants would exhibit only significant prefrontal-to-amygdala connectivity (owing to limbic hypo-responsiveness7); and (iii) AN participants would not exhibit significant fronto-limbic connectivity (suggesting impaired top-down modulation). These hypotheses pertain to the block-to-block within-group increase in connectivity. Finally, we predicted that fronto-limbic connectivity would be associated with anxiety symptoms, and core AN and BDD symptoms.Methods
Ninety-four unmedicated young adults were recruited (BDD=32, AN=25 weight restored, controls=37, matched in age, gender, education and BMI). FMRI data was acquired in a Siemens Trio 3T scanner (EPI sequence, TR/TE=2500/25ms, flip-angle=80o, FOV=192mm, voxel-size=3×3×3mm3, slice-gap=0.75mm). Fearful face stimuli were presented in a blocked design: four unique stimuli (4s each with 500ms inter-stimulus interval) per block for three blocks, to assess habituation. Participants indicated how fearful they felt (subjective fear rating), or the shape of scrambled face as the control task. Standard pre-processing was performed in FSL10 (motion correction [6-parameters], spatial smoothing [5mm-kernel], high-pass filtering [100s], co-registration to MNI-space). Eigenvariate timeseries from bilateral mPFC, amygdala (both Harvard-Oxford atlas) and rACC (Freesurfer) were extracted. Hemodynamic deconvolution was performed11 to minimize the non-neural intra-subject HRF variability12. Dynamic effective connectivity (DEC) was evaluated between all ROI pairs by employing Kalman-filter based time-varying Granger causality13, which provided the connectivity value at every time-point. Mean DEC values corresponding to each of the three fearful-face blocks were extracted separately for all connections. We then sought to identify those connections that increased significantly across the blocks, indicating enhanced engagement, which would be expected to facilitate habituation. Specifically, we looked for connections exhibiting this DEC profile: block3>block2 AND block2>block1 (p<0.05, Bonferroni corrected, for both comparisons). This procedure was performed separately for the three groups to test our three hypotheses. Association between significant connectivities with relevant clinical variables were tested (subjective fear rating (SFR), eating-disorder examination score [EDE] and Hamilton anxiety rating scale [HAMA]).Results and Discussion
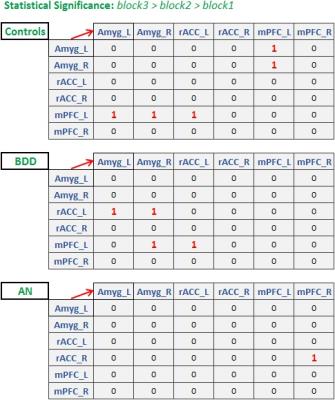
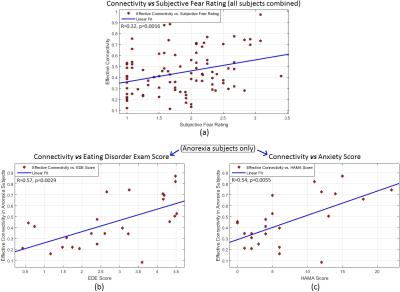
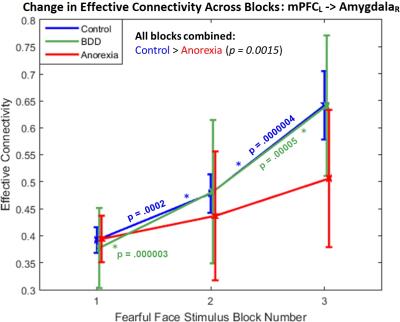
Fig.1 shows the significant connections identified as increasing progressively across the three task blocks. We found evidence for our hypotheses. There was significant mPFC-amygdala bidirectional connectivity in controls, only unidirectional mPFC-to-amygdala connectivity in BDD, and no prefrontal-amygdala connectivity in AN. There was a significant interaction between block and group, with AN showing weaker increases in connectivity than controls across the blocks (Fig. 2). Thus, impaired fronto-limbic modulation might distinguish AN from BDD and controls. In BDD, while top-down fronto-limbic modulation was not entirely disrupted, we found abnormalities suggesting that BDD participants might employ alternate strategies to facilitate habituation. The mPFC-to-amygdala connectivity is known to play a pivotal role in prefrontal top-down regulation during habituation9. Our findings suggest an efficient two-way communication paradigm between mPFC and amygdala in healthy controls. This appears to be demonstrably disrupted in AN, which is consistent with the prominent clinical anxiety phenotype in AN. Moreover, we found that mPFC-to-amygdala connectivity (averaged across all blocks) had significant association with (see Fig.2) SFR across all participants (R=0.32,p=0.0016), with EDE in AN (R=0.57,p=0.0029), and with HAMA in AN (R=0.54,p=0.0055). These observations ascribe clinical relevance to the mPFC-to-amygdala connection. In summary, there is evidence of distinct abnormal fronto-limbic fear circuitry in AN and BDD. In AN, both anxiety and eating disorder symptoms may be a function of abnormal emotional expression and/or regulation. This work contributes to a mechanistic understanding of the neural substrates underlying the similarities and differences in AN and BDD, and may have important clinical relevance regarding treatments that engage fronto-limbic circuitry.Acknowledgements
No acknowledgement found.References
[1] Veale D. et al., "Body dysmorphic disorder in different settings: A systematic review and estimated weighted prevalence." Body Image.;18:168-186;2016
[2] Hudson JI, Hiripi E, Pope HG Jr, Kessler RC, "The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication", Biol Psychiatry;61:348–358;2007
[3] Phillips KA, "The Broken Mirror: Understanding and Treating Body Dysmorphic Disorder", Oxford University Press (2005)
[4] Association AP, "Diagnostic and statistical manual of mental disorders, (DSM-5®)", American Psychiatric Pub. (2013)
[5] Li W, Lai TM, Bohon C, Loo SK, McCurdy D, Strober M, et al, "Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information", Psychol Med.;45(10):2111-22;2015
[6] Strober M, Freeman R, Lampert C, Diamond J, "The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications", Int J Eat Disord;40:S46-51;2007
[7] Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, et al., "Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder", Arch Gen Psychiatry;67:197-205;2010
[8] Vuilleumier P, Pourtois G, "Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging", Neuropsychologia;45:174-194;2007
[9] Quirk GJ, Mueller D, "Neural mechanisms of extinction learning and retrieval", Neuropsychopharmacology;33:56-72;2007
[10] http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/ (accessed on 9-Nov-2016)
[11] Havlicek M, Friston KJ, Jan J, Brazdil M, Calhoun VD, "Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering", Neuroimage;56(4):2109-28;2011
[12] Handwerker DA, Ollinger JM, D'Esposito M, "Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses", Neuroimage;21(4):1639-51;2004
[13] Büchel C, Friston KJ, "Dynamic changes in effective connectivity characterized by variable parameter regression and Kalman filtering", Human Brain Mapping;403-408;1998
Figures