4213
Connectome-wide exploration of altered resting-state connectivity in combat veterans with and without PTSD and real-time fMRI neurofeedback training effect on abnormal connectivity1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Department of Psychology, George Mason University, Fairfax, VA, United States, 3Deptartment of Psychological Science, University of Arkansas, Fayetteville, AR, United States, 4College of Engineering, Stephenson School of Biomedical Engineering, University of Oklahoma, Tulsa, OK, United States
Synopsis
In combat veterans with and without PTSD diagnosis, we performed connectome-wide exploration of the whole-brain voxel-by-voxel fMRI connectivity using multivariate distance-based matrix regression (MDMR) analysis to determine connectivity abnormalities without a priori hypothesis. PTSD veterans showed increased connectivity across sensory motor areas and the superior temporal to default mode network (DMN) areas compared to non-trauma-exposed control. Veterans without PTSD also showed altered connectivity in the bilateral insula compared to control. This abnormal connectivity pattern was normalized after real-time fMRI neurofeedback training focused on learning to control left amygdala activity with positive autobiographical memory recall.
PURPOSE
Altered resting-state fMRI connectivity has been reported in posttraumatic stress disorder (PTSD)1, but many studies have used a-priori-defined seed-based analysis or global network pattern analysis (e.g., ICA), which could have missed a critical alteration in an unpredicted region. We performed an unbiased search for whole-brain voxel-by-voxel connectivity alterations in combat veterans with and without PTSD using multivariate distance-based matrix regression (MDMR) analysis (connectome-wide association study)2. We also examined the effect of emotion regulation training with real-time fMRI neurofeedback (rtfMRI-nf) on identified abnormal connectivity.METHODS
Participants: Thirty-five male combat veterans with PTSD (age 21-48) and 18 male combat veterans without PTSD (veterans control, VC; age 22-55) participated in the resting-state fMRI scan before the rtfMRI-nf training sessions. Resting-state fMRI data of 28 age-matched non trauma-exposed healthy males who participated in a separate study were used as controls (non-trauma controls, NC; age 19-53). Of veteran subjects, 22 with PTSD and 11 VCs participated in 3 days of rtfMRI-nf training and completed a post-training rest scan. During the rtfMRI-nf procedure, subjects were trained to increase left amygdala activity while recalling positive autobiographical memory with rtfMRI-nf3. Six subjects with PTSD received feedback of intraparietal activity as a sham control group (PTSD sham).
fMRI preprocessing: AFNI was used for image processing. Physiological noise reduction with RETROICOR/RVT, slice-timing and motion correction, nonlinear warping to the MNI template brain, spatial smoothing (4mm-FWHM), and scaling to percent change were applied to resting-state fMRI data. Noise in signal time-course was removed by regressing out three principal components of ventricle signal, local white matter average signal (ANATICOR4), motion parameters, and low-frequency fluctuation and censoring volumes with large head motion.
MDMR analysis overview: The following was completed for each subject: a) down-sampled fMRI images to 4mm3 voxels; b) extracted voxels in gray matter region; c) calculated correlation of signal time-courses for each voxel to all other brain voxels and applied Fisher Z-transform to make a connectivity map for each voxel; d) calculated Euclid distance between connectivity maps of the subjects to make a distance matrix; e) applied nonparametric MANCOVA for the distance matrix. P-value was evaluated by a 10,000-repetition permutation test. These steps were repeated for each voxel as a seed to make a statistical parametric map.
RESULTS
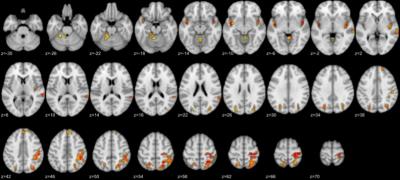
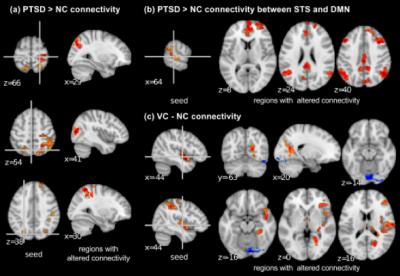
Figure 1 shows the regions that differed between PTSD, VC, and NC groups in the MDMR analysis. These regions were used as seeds for post-hoc analysis. Post-hoc analysis revealed that PTSD compared to NC had increased connectivity across sensory motor regions including the precentral gyrus, the intraparietal region, and the precuneus region (Figure 2a). PTSD also showed increased connectivity between the superior temporal sulcus (STS) and the DMN regions compared to NC (Figure 2b). VC subjects compared to NC showed altered connectivity from the bilateral posterior insula (Figure 2c). No significant difference was seen between PTSD and VC groups.
All of the abnormal functional connectivity identified in
the previous analysis showed significant normalization after the rtfMRI-nf
training. Hyperconnectivity in sensory motor areas and the STS to the DMN
regions for PTSD were reduced after the training (Figure 3a). While the effect
was seen for both PTSD active (receiving amygdala activity feedback) and PTSD
sham groups, the active group showed more significant reduction (P=0.001) than the sham group (P=0.039). Importantly only the PTSD active group showed
significant reduction of PTSD symptoms measured by PCL-M after the training (P=0.005). Abnormal connectivity in VC was also
normalized after the training (Figure 3b) though no symptom change was
observed.
DISCUSSION
Veterans with PTSD had greater connectivity across sensory motor areas, which could be associated with hyperarousal symptoms in PTSD. Connectivity between the right superior temporal region and the DMN regions also was increased in PTSD, which might be associated with dissociation symptoms in PTSD. It has been indicated that abnormal activity in the superior temporal region is associated with dissociation symptoms in PTSD5 and the DMN is related to introspective thinking6. VC subjects also showed altered connectivity in the bilateral posterior insula. While VC subjects showed no PTSD symptoms, military training and combat experience might leave some effects on brain functional connectivity.
These abnormal connectivity patterns were normalized after the rtfMRI-nf training. As the normalization was seen both for active and sham feedback groups, the training experience itself could have a positive effect on PTSD. However, and importantly, the active feedback group, showed a more pronounced training effect than the sham group, which indicated that the neurofeedback helped to enhance the positive effect of the training.
Acknowledgements
This study was supported by the W81XWH-12-0697 grant from the U.S. Department of Defense.References
1. Wang T, Liu J, Zhang J et al. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Scientific reports 2016;6:27131.
2. Shehzad Z, Kelly C, Reiss PT et al. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 2014;93 Pt 1:74-94.
3. Zotev V, Krueger F, Phillips R et al. Self-Regulation of Amygdala Activation Using Real-Time fMRI Neurofeedback. PLoS ONE 2011;6(9):e24522.
4. Jo HJ, Saad ZS, Simmons WK et al. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 2010;52(2):571-582.
5. Lanius RA, Bluhm R, Lanius U et al. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. Journal of psychiatric research 2006;40(8):709-729.
6. Hamilton JP, Farmer M, Fogelman P et al. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry 2015.
Figures