4198
Relation between brain temperature and cerebral perfusion and metabolism in human brain1Department of Neurosurgery, Iwate Medical University, Morioka, Japan, 2WPI Immunology Frontier Research Center, Osaka University, Suita, Japan, 3Department of Hyperbaric Medicine, Iwate Medical University, Morioka, Japan, 4Center for Information and Neural Networks (CiNet), NICT and Osaka University, Suita, Japan
Synopsis
Brain temperature (BT) had traditionally been discussed whether it may be a simple parameter depending on body (core) temperature or it may regulate the neural activities; however, recent reports with magnetic resonance imaging (MRI) system demonstrated BT was strongly associated with the cerebral perfusion and metabolism in patients with ischemic change. Here, we reviewed BT measurement techniques with a MRI system and discussed the pathologic conditions causing BT alteration relating to the cerebral perfusion and metabolism.
PURPOSE
Brain temperature (BT) had traditionally been discussed whether it may be a simple parameter depending on body (core) temperature or it may regulate the neural activities. Recent reports with magnetic resonance (MR) imaging system demonstrated BT was strongly associated with the cerebral perfusion and metabolism in patients with ischemic change. Based on these results, we should refocus and reconsider on BT and the alteration mechanism using MR. Here, we reviewed BT measurement techniques with MR and discussed the pathologic conditions causing BT alteration relating to the cerebral perfusion and metabolism.Outline of content
Direct measurements: About direct BT measurements in patients, a few groups have demonstrated the interesting results1-4. Shiraki, et al. may publish the first report of BT measurement. They performed the direct measurement by a drainage catheter in a patient who underwent surgery to relieve intracranial pressure by a pineal tumor, and they showed the independency between BT and tympanic temperature1. The other group also performed the measurement by the ventricular catheter in patients with subarachnoid hemorrhage (SAH)4. This group demonstrated that the higher BT group showed good outcome aſter SAH and all patients in the lower BT group died. This research also showed that the outcome became worse when BT decreased less than the systemic temperature. In these results, the lower BT might indicate the remarkably low metabolism.
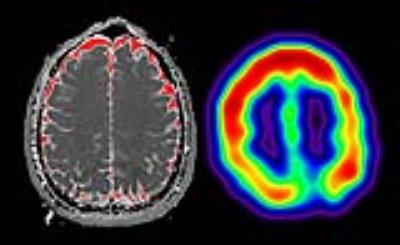
Non-invasive measurements: 1H-MRS, which can be non-invasively performed with multi-voxel scan as well as single voxel scan, has been used for temperature measurements in human (Figure 1)5-11. Cady et al. performed the BT measurement of newborn infants5. By the calibrated date, they defined a basic formula to estimate the temperature using the amount of the chemical shift between H2O and NAA as follows: T [°C] = 286.9-94×Δ(H2O-NAA). This formula has been a key to measure BT with 1H-MRS in recent researches9-11. Yablonskiy, et al. demonstrated that the relation between temperature and blood flow in the local area in the brain by using functional MRI and 1H-MRS6; however, they could not show whether BT was associated with the oxygen metabolism directly measured by a gold standard modality like positron emission tomography (PET) to assess the cerebral perfusion and metabolism. Ishigaki, et al. directly compared the BT by 1H-MRS with the parameters on PET in patients with chronic ischemia9. Their results showed BT significantly correlated with oxygen extraction fraction (OEF) and BT can detect patients with OEF elevation relating to a high risk of stroke recurrence. By this key paper, BT alteration might be quantitatively connected with the cerebral perfusion and metabolism. Carbon monoxide (CO) poisoning can cause abnormal OEF elevation12, which means that cerebral blood flow (CBF) reduced and cerebral metabolic rate of oxygen (CMRO2) maintained. In recent reports, abnormal BT elevation was observed during the period between acute and subacute phase after CO exposure13,14. On the other hand, BT significantly decreased at the subacute phase comparing with the acute, in particular, BT in patients with severe white matter damage mimicked the normal14. The results may indicate that the brain metabolism to produce heat for BT decreased by severe white matter damage observed in patients with severe CO poisoning. These results in patients with CO poisoning indicate that BT alteration is strongly associated with the cerebral metabolism. Diffusion weighted imaging (DWI) can be also used for BT measurements. Yamada, et al. demonstrated the difference between healthy controls and patients with moyamoya disease, which is a kind of chronic ischemia13. Fujiwara, et al. tried to measure BT in patients with CO poisoning with DWI and the temperature in the arachnoid space measured by DWI significantly correlated with that in the white matte region measured by 1H-MRS-BT14 (Figure 2). DWI temperature measurement potentially may help us to know the BT alteration in the lesion with the difficulty in such measurement with 1H-MRS.
Summary
We reviewed techniques for BT measurement and the relation between BT and the cerebral perfusion and metabolism. We can sensitively demonstrate the subtle temperature change relating to the basic system in the brain as well as static anatomy, susceptibility change and dynamic function with statistical analysis.Acknowledgements
This study was supported in part by Grant-in-Aid for Scientific Research (C) (No.15K09935, 2015-2018) and Grant-in-Aid for Strategic Medical Science Research (S1491001, 2014-2018) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
1. Shiraki K, Sagawa S, Tajima F, Yokota A, Hashimoto M, Brengelmann GL. Independence of brain and tympanic temperatures in an unanesthetized human. J Appl Physiol. 1988;65(1):482-6.
2. Mellergard P. Intracerebral temperature in neurosurgical patients: intracerebral temperature gradients and relationships to consciousness level. Surg Neurol. 1995;43(1):91-5.
3. Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998;26(3):562-7.
4. Otawara Y, Ogasawara K, Kubo Y, Tomitsuka N, Ogawa A, Suzuki M. Brain and systemic temperature in patients with severe subarachnoid hemorrhage. Surg Neurol. 2003;60(2):159-64; discussion 64.
5. Cady EB, D'Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1995;33(6):862-7.
6. Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci U S A. 2000;97(13):7603-8.
7. Yoshioka Y, Oikawa H, Ehara S, Inoue T, Ogawa A, Kanbara Y, et al. Noninvasive measurement of temperature and fractional dissociation of imidazole in human lower leg muscles using 1H-nuclear magnetic resonance spectroscopy. J Appl Physiol. 2005;98(1):282-7.
8. Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol. 2006;60(4):438-46.
9. Ishigaki D, Ogasawara K, Yoshioka Y, Chida K, Sasaki M, Fujiwara S, et al. Brain temperature measured using proton MR spectroscopy detects cerebral hemodynamic impairment in patients with unilateral chronic major cerebral artery steno-occlusive disease: comparison with positron emission tomography. Stroke. 2009;40(9):3012-6.
10. Fujiwara S, Yoshioka Y, Matsuda T, Nishimoto H, Murakami T, Ogawa A, et al. Brain temperature measured by 1H-magnetic resonance spectroscopy in acute and subacute carbon monoxide poisoning. Neuroradiology. 2016;58(1):27-32.
11. Fujiwara S, Yoshioka Y, Matsuda T, Nishimoto H, Ogawa A, Ogasawara K, et al. Relation between brain temperature and white matter damage in subacute carbon monoxide poisoning. Sci Rep. 2016;6:36523.
12. De Reuck J, Decoo D, Lemahieu I, Strijckmans K, Boon P, Van Maele G, et al. A positron emission tomography study of patients with acute carbon monoxide poisoning treated by hyperbaric oxygen. J Neurol. 1993;240(7):430-4.
13. Yamada K, Sakai K, Akazawa K, Yuen S, Sugimoto N, Sasajima H, et al. Moyamoya patients exhibit higher brain temperatures than normal controls. Neuroreport. 2010;21(13):851-5.
14. Fujiwara S, Yoshioka Y, Matsuda T, Nishimoto H, Murakami T, Ogawa A, et al. Diffusion-weighted thermometry using subarachnoid space cerebrospinal fluid in subacute carbon monoxide poisoning patients. ISMRM 23rd Annual Meeting & Exhibition, Toronto, Canada, 30 May – 5 June, 2015.
Figures

Figure 1 Relation between the chemical shift of H2O and temperature (left) and a brain temperature map by the chemical shift imaging based on magnetic resonance spectroscopy.