4196
Magnetic Resonance Imaging of Myelin Water: Principles and Applications1Radiology, University of British Columbia, Vancouver, BC, Canada, 2Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 3International Collaboration on Repair Discoveries, University of British Columbia, Vancouver, BC, Canada, 4Medicine, University of British Columbia, Vancouver, BC, Canada, 5Interior Health, Kelowna, BC, Canada, 6Computer Science, Mathematics, Physics & Statistics, University of British Columbia Okanagan, Kelowna, BC, Canada, 7Physics & Astronomy, University of British Columbia, Vancouver, BC, Canada, 8Kinesiology, University of British Columbia, Vancouver, BC, Canada, 9Pediatrics, University of British Columbia, Vancouver, BC, Canada, 10Physical Therapy, University of British Columbia, Vancouver, BC, Canada
Synopsis
Myelin water imaging (MWI) provides quantitative and specific mapping of myelin content in-vivo. Water trapped between myelin bilayers have a short T2 relaxation time; the fractional proportion of this myelin water signal correlates strongly with histological staining for myelin. MWI has successfully been used to study both the brain and spinal cord where it can increase our understanding of development, aging and disease processes, and may also improve accuracy of diagnosis, prognosis and assessment of therapeutic response. Moving forward, MWI is expected to play an important role in the development and monitoring of new treatments targeted at remyelination and neuroprotection.
Purpose
To provide an overview of the principles and applications of myelin water imaging in neurological disorders. The specific objectives are to:
1. Explain the anatomical basis of myelin water.
2. Describe the MRI acquisition and analysis of multi-echo T2-relaxation based myelin water imaging.
3. Summarize key myelin water imaging findings in healthy brain and spinal cord tissue.
4. Review myelin water imaging abnormalities in different central nervous system (CNS) developmental and acquired pathological conditions
5. Learn how myelin water findings compare to other quantitative neuroimaging MR techniques such as diffusion tensor, magnetization transfer imaging and frequency shift imaging.
Outline of Content
1. What is the value of measuring myelin?
2. Background on myelin water imaging technique (a. anatomical basis of myelin water, b. data acquisition, c. data analysis)
3. Is myelin water actually related to myelin? Histological validation in preclinical models and post-mortem human tissue
4. Myelin water in healthy tissue (a. brain, b. spinal cord)
5. Myelin water in pathological or abnormal tissue (a. multiple sclerosis, b. neuromyelitis optica, c. stroke, d. schizophrenia, e. autism, f. primary and amyotrophic lateral sclerosis, g. concussion, h. phenylketonuria, i. neurofibromatosis, j. Niemann-Pick Disease, k. spinal cord injury)
6. Comparison of myelin water to other quantitative neuroimaging methods
7. What is the significance and future of myelin water imaging?
Summary
Accurately measuring myelin in-vivo will improve our understanding of development, aging and neurological diseases as well as enable better assessment of myelin-targeted therapies. Although conventional MRI can detect pathological changes, it lacks specificity for the type(s) of tissue injury and cannot detect abnormalities in the so-called normal appearing white matter. More sophisticated MRI techniques can provide more specific information about myelin content in the CNS. MRI signal in CNS tissue arises almost entirely from water in different physical environments with unique T2 relaxation times1, 2. Water trapped between myelin bilayers (myelin water, Figure 1) has a short T2, while intra/extra-cellular water has a longer T2. The amount of water in each environment can be measured using a multi-echo spin-echo approach, which samples the MRI signal multiple times during T2 relaxation3-9. A fit of the T2 decay curve (signal vs. time) determines the size of the different water environments10. Several other approaches to myelin water measurement and analysis have also been proposed11-22.
Pathology-MRI validation in preclinical models and post-mortem multiple sclerosis CNS tissue (Figure 2) show excellent quantitative agreement between multi-echo derived myelin water content and histological staining for myelin2, 23-30. Myelin water imaging has successfully demonstrated changes with development31-35, regional variation in normal brain (Figure 3)3, 36 and spinal cord37-39, and myelin abnormalities in multiple sclerosis (Figure 4)10, 40-43, neuromyelitis optica44, stroke (Figure 4)45, schizophrenia46, 47, autism48, dyslexia49, dyscalculia50, primary/amyotrophic lateral sclerosis51, concussion52, phenylketonuria53, neurofibromatosis54, Niemann-Pick Disease55, and spinal cord injury (Figure 5)56, in both cross-sectional and longitudinal studies. Several clinical trials have included myelin water as an outcome measure57, 58
Several other quantitative MR methods are also influenced by myelin, including magnetization transfer ratio (MTR), diffusion tensor imaging (DTI), frequency shift imaging (FSI) and magnetic resonance spectroscopy (MRS). However, the lack of strong correlation between MTR, DTI and FSI and myelin water suggest these measures provide complementary information; the correlation between MRS and myelin water is currently being investigated59-61.
In summary, myelin water is a specific imaging biomarker that provides quantitative mapping of myelin content in-vivo. It can increase our understanding of development, aging, disease processes and may improve accuracy of diagnosis, prognosis and assessment of therapeutic response. Moving forward, myelin water imaging is expected to play an important role in the development and monitoring of new treatments targeted at remyelination and neuroprotection.
Acknowledgements
We sincerely thank all participants for studies done locally at our centre, as well as the MRI technologists and administrator. Funding support for work includes Multiple Sclerosis Society of Canada, NSERC, CIHR, Michael Smith Foundation for Health Research, the Cervical Spine Research Society, London Drugs Award for Research Excellence, a Vancouver Hospital and Health Sciences Centre Interdisciplinary Grant, and a seed grant from the International Collaboration on Repair Discoveries.References
[1] Fischer, H.W., et al., Nuclear relaxation of human brain gray and white matter: analysis of field dependence and implications for MRI. Magnetic Resonance in Medicine., 1990. 16(2): p. 317-34.
[2] Stewart, W.A., et al., Spin-spin relaxation in experimental allergic encephalomyelitis. Analysis of CPMG data using a non-linear least squares method and linear inverse theory. Magnetic Resonance in Medicine., 1993. 29(6): p. 767-75.
[3] MacKay, A., et al., In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine, 1994. 31(6): p. 673-7.
[4] Wu, Y., et al., Myelin water fraction in human cervical spinal cord in vivo. J Comput Assist Tomogr., 2006. 30(2): p. 304-6.
[5] Prasloski, T., et al., Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage, 2012. 63(1): p. 533-9.
[6] Oh, J., et al., Multislice brain myelin water fractions at 3T in multiple sclerosis. Journal of Neuroimaging, 2007. 17(2): p. 156-63.
[7] Oh, J., et al., Measurement of in vivo multi-component T2 relaxation times for brain tissue using multi-slice T2 prep at 1.5 and 3 T. Magn Reson Imaging., 2006. 24(1): p. 33-43. Epub 2005 Dec 19.
[8] Nguyen, T.D., et al., T(2) prep three-dimensional spiral imaging with efficient whole brain coverage for myelin water quantification at 1.5 tesla. Magnetic Resonance in Medicine, 2012. 67(3): p. 614-21.
[9] Nguyen, T.D., et al., Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med, 2015.
[10] Whittall, K.P., et al., In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine, 1997. 37(1): p. 34-43.
[11] Du, Y.P., et al., Fast multislice mapping of the myelin water fraction using multicompartment analysis of T2* decay at 3T: a preliminary postmortem study. Magn Reson Med, 2007. 58(5): p. 865-70.
[12] Sati, P., et al., Micro-compartment specific T2* relaxation in the brain. Neuroimage, 2013. 77: p. 268-78.
[13] Nam, Y., et al., Improved estimation of myelin water fraction using complex model fitting. Neuroimage, 2015. 116: p. 214-21.
[14] Lenz, C., M. Klarhofer, and K. Scheffler, Feasibility of in vivo myelin water imaging using 3D multigradient-echo pulse sequences. Magnetic Resonance in Medicine, 2012 Aug;68(2):523-8.
[15] Deoni, S.C., et al., Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med, 2008. 60(6): p. 1372-87.
[16] Stanisz, G.J. and R.M. Henkelman, Diffusional anisotropy of T2 components in bovine optic nerve. Magn Reson Med., 1998. 40(3): p. 405-10.
[17] Raj, A., et al., Multi-compartment T2 relaxometry using a spatially constrained multi-Gaussian model. PLoS One, 2014. 9(6): p. e98391.
[18] Kumar, D., et al., Bayesian algorithm using spatial priors for multiexponential T(2) relaxometry from multiecho spin echo MRI. Magn Reson Med, 2012. 68(5): p. 1536-43.
[19] Shen, X., et al., Robust myelin quantitative imaging from multi-echo T2 MRI using edge preserving spatial priors. Med Image Comput Comput Assist Interv, 2013. 16(Pt 1): p. 622-30.
[20] Akhondi-Asl, A., et al., T(2)-relaxometry for myelin water fraction extraction using wald distribution and extended phase graph. Med Image Comput Comput Assist Interv, 2014. 17(Pt 3): p. 145-52.
[21] Guo, J., Q. Ji, and W.E. Reddick, Multi-slice myelin water imaging for practical clinical applications at 3.0 T. Magn Reson Med, 2013. 70(3): p. 813-22.
[22] Yoo, Y. and R. Tam. Non-local spatial regularization of MRI T2 relaxation images for myelin water quantification. in Medical Image Computing and Computer Assisted Intervention (MICCAI) Part 1. 2013. p.614-621
[23] Gareau, P.J., et al., In vivo measurements of multi-component T2 relaxation behaviour in guinea pig brain. Magnetic Resonance Imaging., 1999. 17(9): p. 1319-25.
[24] Kozlowski, P., et al., High-resolution myelin water measurements in rat spinal cord. Magn Reson Med, 2008 Apr;59(4):796-802.
[25] McCreary, C.R., et al., Multiexponential T2 and magnetization transfer MRI of demyelination and remyelination in murine spinal cord. Neuroimage, 2009. 45(4): p. 1173-82.
[26] Kozlowski, P., et al., In vivo longitudinal Myelin Water Imaging in rat spinal cord following dorsal column transection injury. Magn Reson Imaging, 2014. 32(3): p. 250-8.
[27] Moore, G.R.W., et al., A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology, 2000. 55(10): p. 1506-10.
[28] Laule, C., et al., Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler., 2006. 12(6): p. 747-53.
[29] Laule, C., et al., Myelin water imaging of multiple sclerosis at 7 T: Correlations with histopathology. Neuroimage, 2008. 40: p. 1575-1580.
[30] Laule, C., et al., High-resolution myelin water imaging in post-mortem multiple sclerosis spinal cord: A case report. Mult Scler, 2016 Oct;22(11):1485-1489.
[31] Deoni, S.C., et al., Mapping infant brain myelination with magnetic resonance imaging. Journal of Neuroscience, 2011. 31(2): p. 784-91.
[32] Dean, D.C., 3rd, et al., Modeling healthy male white matter and myelin development: 3 through 60months of age. Neuroimage, 2014. 84: p. 742-52.
[33] Dean, D.C., 3rd, et al., Characterizing longitudinal white matter development during early childhood. Brain Struct Funct, 2015. 220(4): p. 1921-33.
[34] Deoni, S.C., et al., Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage, 2012. 63(3): p. 1038-53.
[35] Whitaker, K.J., et al., Quantifying development: Investigating highly myelinated voxels in preadolescent corpus callosum. Neuroimage, 2008 Dec;43(4):731-5.
[36] Liu, F., et al., Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Research, 2010. 1343: p. 37-45.
[37] Minty, E.P., et al., Myelin water measurement in the spinal cord. Magn Reson Med, 2009. 61(4): p. 883-92.
[38] Ljungberg, E., et al. Rapid Myelin Water Imaging in Human Cervical Spinal Cord in International Society of Magnetic Resonance in Medicine. 2016. Singapore. p. 282.
[39] MacMillan, E.L., et al., Myelin water and T(2) relaxation measurements in the healthy cervical spinal cord at 3.0T: repeatability and changes with age. NeuroImage, 2011. 54(2): p. 1083-90.
[40] Laule, C., et al., Water content and myelin water fraction in multiple sclerosis: A T2 relaxation study. Journal of Neurology, 2004. 251(3): p. 284-293.
[41] Vavasour, I.M., et al., A comparison between magnetization transfer ratios and myelin water percentages in normals and multiple sclerosis patients. Magnetic Resonance in Medicine., 1998. 40(5): p. 763-8.
[42] Laule, C., et al., Two-year study of cervical cord volume and myelin water in primary progressive multiple sclerosis. Mult Scler, 2010. 16(6): p. 670-7.
[43] Vavasour, I.M., et al., Longitudinal changes in myelin water fraction in two MS patients with active disease. J Neurol Sci, 2009. 276(1-2): p. 49-53.
[44] Jeong, I.H., et al., Comparison of myelin water fraction values in periventricular white matter lesions between multiple sclerosis and neuromyelitis optica spectrum disorder. Mult Scler, 2016 Oct;22(12):1616-1620.
[45] Borich, M.R., et al., Evaluation of white matter myelin water fraction in chronic stroke. Neuroimage Clin, 2013. 2: p. 569-80.
[46] Flynn, S.W., et al., Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Molecular Psychiatry, 2003. 8(9): p. 811-20.
[47] Lang, D.J., et al., 48 echo T2 myelin imaging of white matter in first-episode schizophrenia: Evidence for aberrant myelination. Neuroimage Clin, 2014. 6: p. 408-14.
[48] Deoni, S.C., et al., White-matter relaxation time and myelin water fraction differences in young adults with autism. Psychol Med, 2015. 45(4): p. 795-805.
[49] Yip, E., et al. Myelin Water Imaging of Children with Diverse Reading Ability. in International Society for Magnetic Resonance in Medicine. 2010. Stockholm, Sweden. p. 2346.
[50] Holmes, R.D., et al. Cerebral Myelin Content Correlation with Mathematical Abilities in Young Children. in International Society for Magnetic Resonance in Medicine. 2010. Stockholm, Sweden. p. 6.
[51] Kolind, S., et al., Myelin imaging in amyotrophic and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener, 2013. 14(7-8): p. 562-73.
[52] Wright, A.D., et al., Myelin Water Fraction Is Transiently Reduced after a Single Mild Traumatic Brain Injury - A Prospective Cohort Study in Collegiate Hockey Players. PLoS One, 2016. 11(2): p. e0150215.
[53] Sirrs, S.M., et al., Normal appearing white matter in subjects with phenylketonuria: water content, myelin water fraction, and metabolite concentrations. Radiology, 2007. 242(1): p. 236-243.
[54] Billiet, T., et al., Characterizing the microstructural basis of "unidentified bright objects" in neurofibromatosis type 1: A combined in vivo multicomponent T2 relaxation and multi-shell diffusion MRI analysis. Neuroimage Clin, 2014. 4: p. 649-58.
[55] Davies-Thompson, J., et al., Reduced Myelin Water in the White Matter Tracts of Patients with Niemann-Pick Disease Type C. AJNR Am J Neuroradiol, 2016 Aug;37(8):1487-9.
[56] Liu, H., et al. Assessing Structure and Function of Myelin in Cervical Spondylotic Myelopathy: Evidence of Focal Demyelination in the Dorsal Column in International Society of Magnetic Resonance in Medicine. 2016. Singapore. p. 849.
[57] Vavasour, I.M., et al. Advanced imaging in lesion and normal-appearing white matter over 2 years in MS patients treated with alemtuzumab. in International Society for Magnetic Resonance in Medicine. 2015. Toronto, Canada. p. 4336.
[58] Vavasour, I.M., et al. Comparison of Three Putative MR Myelin Markers in Multiple Sclerosis Subjects and Healthy Controls. in International Society for Magnetic Resonance in Medicine. 2014. Milano, Italy. p. 2066.
[59] Hill, R.A., Do short-term changes in white matter structure indicate learning-induced myelin plasticity? J Neurosci, 2013. 33(50): p. 19393-5.
[60] Mädler, B., et al., Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging, 2008. 26(7): p. 874-88.
[61] Vavasour, I.M., et al., Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J Magn Reson Imaging, 2011. 33(3): p. 713-8.
Figures
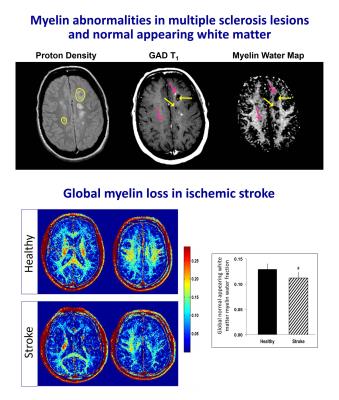
Figure 4 - Myelin water imaging can monitor pathology in diseases where myelin is damaged such multiple sclerosis (MS) and stroke.
Top: MS lesions show heterogeneous reductions (pink arrows: 2 areas of T1 enhancement, one area has myelin loss while the other does not; yellow arrows: T1 black-holes show different degrees of myelin loss beyond the T1 hypointensity). Normal appearing white matter has 16% less myelin than controls.40
Bottom: Ischemic stroke demonstrates white matter myelin loss compared to controls, including global normal appearing white matter: -13.8%; ipsilesional posterior limb of internal capsule: -14.9%; and contralateral posterior limb of internal capsule: -10.0%.45