4191
State of the art stroke imaging1Neuroradiology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
This work will review key concepts of our current understanding of stroke pathophysiology, imaging protocol with an interest on specific MRI sequences. This review will also introduce important concepts regarding stroke-related neuroinflammation.
Purpose
Review up do date knowledge related to stroke imaging:
- Basic pathophysiology of acute stroke
- State of the art stroke imaging protocols and algorithms
- Recent advances in stroke treatment
- Clinical MR imaging of stroke: DWI, T1, T2, FLAIR, SWI and Perfusion imaging
- MR imaging of neuroinflammation after stroke
Outline of Content
Stokes causes 9% of all death worldwide and is one of the leading cause of disability1. The cost related of this disease represent approximatively 2-4% of the total health-care cost worldwide. Strokes can be either ischemic or hemorrhagic in origin, however, the majority (87%) of these events are ischemic2. Ischemic strokes subdivide further into thrombotic (45%), embolic (20%) and other causes. Recent trials such as MR CLEAN, SWIFT-PRIME, REVASCAT, EXTEND-IA and ESCAPE demonstrated the benefits of intra-arterial (IA) recanalisation therapy. These studies showed that patients treated with IV t-PA + IA treatment had a greater functional independence at 90 days when compared to patients treated with IV t-PA alone.
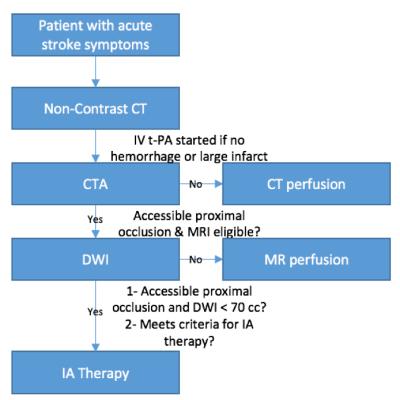
Current therapeutic algorithms rely heavily on CT and CTA to evaluate the presence or absence of hemorrhage and localize the occlusion (Figure 1). However, MRI plays a key role in the stratification of stroke patients and help answering specific questions. For instance, DWI can be used to determine the infarct core size: infarct core greater than 70 ml in volume is an imaging biomarker for poor clinical outcomes, independently of the time of presentation and therapy3. The presence of FLAIR hyperintensity can be used to estimate the age of the stroke: the FLAIR hyperintensity arises 6h after occlusion and rarely before 3h4. Other sequences such as T1 and T2 imaging are also helpful to grade the stroke age. For instance, high signal intensity is not usually seen on T2-weighted imaging until 8 hours after the ischemic insult and low T1-weighted signal is not seen until 16 hours after the onset of stroke5. Perfusion imaging can be used to determine the penumbra, an area of hypoperfusion around the ischemic core causing cellular dysfunction but not cellular death that may be salvage after revascularization. There is currently contradictory studies in the literature regarding the use of the penumbra size to guide endovascular therapy (DEFUSE-2, MR-RESCUE)6,7. This may be partially explained by the lack of standardized MRI techniques between vendor platforms8,9. SWI sequence is useful to detect intra-arterial thrombus, characterize the thrombus (platelet-rich white thrombi versus erythrocytes-rich thrombi)10, a finding that may help differentiate the origin of the thrombus (atherosclerotic versus cardioembolic). Also, some groups believe that SWI may approximate the penumbra11 since a high oxygen extraction fraction is seen in the region of ischemia leading to higher conspicuity of the cortical veins within this area.
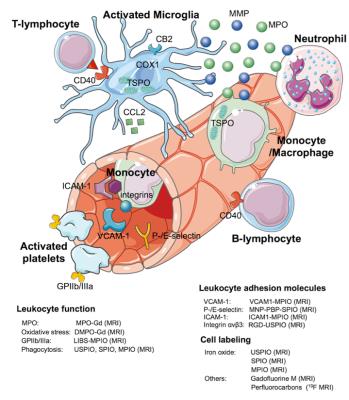
Once the arterial occlusion is removed, other therapeutic targets may be considered, especially in the subacute phase. Indeed, cerebral ischemia and hypoxia lead to direct neuronal injury, however, there is subsequent activation of the inflammatory cascade and microglia that causes secondary neuronal damage. In this work, we will also review current MR imaging of neuroinflammation in ischemic stroke, including imaging leukocyte function, adhesion molecules, and cell labeling (Figure 2). These novel imaging techniques may contribute to the development of new therapies to decrease the propagation and consolidation of stroke secondary to the inflammation process.
Summary
This poster will review key concepts of our current understanding of stroke pathophysiology, imaging protocol with an interest on specific MRI sequences. This review will also introduce important concepts regarding stroke-related neuroinflammation and their importance for future treatments.Acknowledgements
No acknowledgement found.References
1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. The Lancet 2008;371:1612–1623.
2. Go AS, Mozaffarian D, Roger VL, et al. Executive Summary: Heart Disease and Stroke Statistics--2014 Update: A Report From the American Heart Association. Circulation 2014;129:399–410.
3. Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-Based Selection for Intra-Arterial Stroke Therapy: Value of Pretreatment Diffusion-Weighted Imaging Lesion Volume in Selecting Patients With Acute Stroke Who Will Benefit From Early Recanalization. Stroke 2009;40:2046–2054.
4. Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset in acute ischemic stroke patients. Journal of the Neurological Sciences 2010;293:39–44.
5. Yuh WT, Crain MR, Loes DJ, Greene GM, Ryals TJ, Sato Y. MR imaging of cerebral ischemia: findings in the first 24 hours. AJNR Am J Neuroradiol 1991;12:621–629.
6. Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. The Lancet Neurology. 2012;11:860–867.
7. Kidwell CS, Jahan R, Gornbein J, et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. N. Engl. J. Med. 2013;368:914–923.
8. Dani KA, Thomas RGR, Chappell FM, Shuler K, MacLeod MJ, Muir KW, Wardlaw JM, on behalf of the Translational Medicine Research Collaboration Multicentre Acute Stroke Imaging Study. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: Definitions and thresholds. Ann Neurol. 2011;70:384–401.
9. Kamalian S, Kamalian S, Maas MB, Goldmacher GV, Payabvash S, Akbar A, Schaefer PW, Furie KL, Gonzalez RG, Lev MH. CT Cerebral Blood Flow Maps Optimally Correlate With Admission Diffusion-Weighted Imaging in Acute Stroke but Thresholds Vary by Postprocessing Platform. Stroke 2011;42:1923–1928.
10. Cho KH, Kim JS, Kwon SU, Cho A-H, Kang DW. Significance of Susceptibility Vessel Sign on T2*-Weighted Gradient Echo Imaging for Identification of Stroke Subtypes. Stroke 2005;36:2379–2383.
11. Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. Journal of Neuroradiology 2015;42:255–260.