4185
Independent Component-based Denoising for Mapping Cerebrovascular Reactivity with Resting-State Fluctuation of BOLD Signal Amplitude in Patients with GliomasAI-LING HSU1, Ping-Ni Wang2, Jyh-Horng Chen3, and Ho-Ling Liu4
1Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Department of Medical Physics, University of Wisconsin-Madison, 3Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, 4Department of Imaging Physics, The University of Texas MD Anderson Cancer Center
Synopsis
Cerebrovascular reactivity (CVR) with hypercapnia challenges, such as a breath-hold (BH) task, has been proposed to indicate areas with neurovascular uncoupling potentials for presurgical fMRI. Previous studies have shown that BH response correlated with resting-state fluctuation of amplitude (RSFA) in healthy adults. This study explores the use of RSFA for indicating sites with neurovascular uncoupling potentials in presurgical fMRI of patients with gliomas. The RSFA with ICA-based denoising approaches was found to perform superior to the traditional approaches. Unlike BH, RS-fMRI is less dependent on patient performance thus can be widely applied in clinical practice.
PURPOSE
Cerebrovascular reactivity (CVR) MRI with hypercapnia challenges, such as a breath-hold (BH) task, has been proposed to indicate areas with neurovascular uncoupling potentials for presurgical fMRI[1]. Previous studies have shown that BH response correlated with resting-state fluctuation of amplitude (RSFA) in healthy adults[2]. The RSFA could therefore be an alternative for the use in presurgical fMRI when patients have difficulties performing the BH task. However, our preliminary results only showed moderate agreement between the RSFA and the BH-derived CVR results in regions with lesions in glioma patients. In this work, we propose using an independent component analysis (ICA)-based denoising routine combined with the use of RSFA for detecting the impaired CVR in the presurgical fMRI setup.METHODS
The data from five glioma patients were analyzed in the individual space. CVR map were constructed by convolving a HRF with the recorded BH pattern and applying a time delay BH activation, named as CVRBH, was determined by using a threshold of 1.64, corresponding to p < 0.05. The RSfMRI datasets were acquired for 6 minutes using the GEEPI on a 3T clinical scanner with TR/TE=2000/25 ms, and preprocessed with AFNI and FSL using two approaches: (1) RS-fMRI underwent slice-timing correction followed by realignment, detrending, regressing out sources of spurious noise, band-pass filtering (0.01 - 0.08 Hz) and 4 mm-FWHM smoothing. Nuisance regressors included six parameters of head motion, and the fluctuations averaged over two masks of white matter and cerebrospinal fluid, respectively. (2) For second method, after the ICA preprocessing steps, the MELODIC ICA with automatic dimensionality estimation was used to denoise rs-fMRI dataset. The ICA preprocessing includes slice-timing correction, realignment, detrending, non-brain tissue removal, 4 mm-FWHM smoothing, high-pass filtering (0.01 Hz). The IC components were further classified into unlikely artifact components by visual inspection based on the characteristics of spatial pattern, time and frequency information of each IC [3]. After preprocessing, the voxel-wise resting-state fluctuation of BOLD signal amplitude (RSFA) map was measured by calculating the temporal standard deviation of the signal and transforming to z socre. We then referred the RSFA preprocessed by these two approaches as RSFASTD and RSFAICA, respectively. The threshold of RSFA was determined by yielding the same ratio of GM response in BH. For quantitative comparison, a region of interest (ROI) in lesion were produced based on anatomical images of each patient.RESULTS AND DISCUSSION
Figure 1 displays the ratio of GM response in CVR map derived from BH task and the corresponding z threshold of two RSFA maps. The mean overlap of RSFASTD and RSFAICA with respect to CVRBH in the lesion was 59.2% and 60.6%, respectively. The mean overlap of RSFAICA (60.6%,) was non-significantly slightly higher than RSFASTD (59.2%) in the lesion ROI. Furthermore, the false detecting ratio of RSFASTD (36.4%) was significantly (t=2.99, p<0.05) decreased in that of RSFAICA (32.4%). Within individuals, voxel-based analyses showed excellent spatial correspondence with sparse local discrepancies between CVR derived from BH task and both of RSFA approaches (Fig2). Discrepancies between CVRBH and RSFA observed in the lesion was decreased when data processed by ICA denoising.CONCLUSION
The RSFA derived from RS-fMRI is a promising method for probing impaired CVR in presurgical fMRI mapping. Unlike BH, RS-fMRI is less dependent on patient performance. In addition, RSFA preprocessed by ICA denoising approach performed better than by standard approach.Acknowledgements
No acknowledgement found.References
1. Pillai JJ, Mikulis DJ. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am J Neuroradiol. 2015;36: 7–13. doi:10.3174/ajnr.A3941 2. Kannurpatti SS, Motes MA, Rypma B, Biswal BB. Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum Brain Mapp. 2011;32: 1125–1140. doi:10.1002/hbm.21097 3. Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, et al. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189: 233–245. doi:10.1016/j.jneumeth.2010.03.028Figures
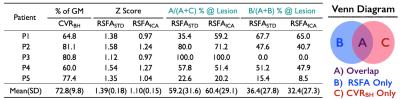
Fig. 1 The ratio of GM response in CVR map derived from BH task and the
corresponding z threshold of two RSFA maps. The correspondence between CVR
derived from BH task and RSFA approaches in lesion ROI.
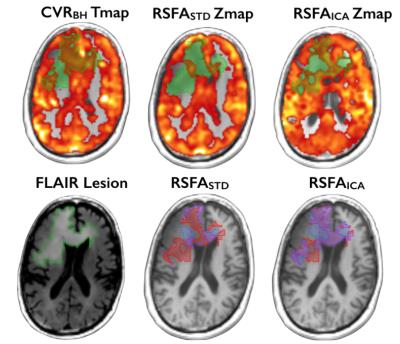
Fig. 2. The spatial pattern among three CVR approaches and their spatial
correspondence in venn diagram.