4176
Preliminary Analysis of Neurite Orientation Dispersion and Density Imaging in Grading of GliomasJing Zhao1, Jian-ping Chu2, Jing-yan Wang2, and Xu Yan3
1The First Affiliated Hospital of Sun Yat-sen University, Guang Zhou, People's Republic of China, 2The First Affiliated Hospital of Sun Yat-sen University, 3Shang Hai
Synopsis
Neurite orientation dispersion and density imaging (NODDI) was an advanced DWI. Our study is to quantitatively evaluate the diagnostic efficiency of NODDI in grading gliomas. 29 patients were recruited and they underwent whole-brain DWI which were collected at three b value (0, 1000 and 2000 s/mm2) and 1000 and 2000 s/mm2 with 30 directions. Compared with LGG, ficvf and ODI are significantly higher in HGG and the mean value of ficvf showed the highest diagnostic value. Quantitative parameters from NODDI can aid in gliomas grading and the mean value of ficvf showed the highest diagnostic power.
Introduction: Preoperative accurate brain tumor diagnosis
plays an essential role in the selection of the optimum treatment strategy, as
their management and prognosis are different. Advanced MRI techniques, such as
DWI, PWI, MRS and DKI, have been wildly used to grading gliomas and the
diagnostic efficiency for grading gliomas has been gradually improved.1-3
Newly developed neurite orientation dispersion and density imaging (NODDI) is
an advanced diffusion weighted imaging and it assumes a three-compartment
biophysical tissue model including intracellular, extracellular, and
cerebrospinal fluids in a single voxel, which enables the inference and
quantification of the direction and structure of neurites (axons and
dendrites).4 Hence, NOODI could apply more information with the changes
of the tumor environment and representative parameters of NODDI are intracellular
volume fraction (ficvf) and orientation dispersion index (ODI).
Nowadays, NODDI has been applied to analyze multiple sclerosis5, focal
cerebral corital dysplasia6 and central nervous system degenerative
disease7 while, barely, NOODI was used to evaluate and grade
gliomas. Therefore, the purpose of our study is to quantitatively evaluate the
diagnostic efficiency of NODDI in tumor parenchyma (TP) and peritumoral (PT) area
for grading gliomas.
Methods: 29 patients (male: 18, female: 11, mean
age: 45.4 y) were prospectively recruited and they underwent conventional, whole-brain
diffusion-weighted images which were collected at three b value (0, 1000 and
2000 s/mm2) and 1000 and 2000 s/mm2 with 30 directions. Both
the b = 1000 s/mm2 and b = 2000 s/mm2 data were used for
the NODDI analysis. Neurite volume fraction (ficvf) and orientation dispersion
index (ODI) maps were generated by post procession software (matlab toolbox).
With each tumor, 6-10 regions of interest (ROIs) were manually placed on TP, PT
and the contralateral normal brain area (CNBA) by Image J. The minimum (min),
mean and maximum (max) values of ficvf and odi and their ratio of TP/CNBA were
calculated and their diagnostic efficiency was assessed by Mann-Whitney test
and ROC analysis.
Results: The mean values of min, mean and max
values of the ficvf and ODI in normal brain were (0.41±0.15), (0.53±0.13),
(0.69±0.18) and (0.23±0.13), (0.37±0.13), (0.55±0.19) respectively. Compared
with CNBA, in TP area, the mean value of ficvf was significantly lower in
glioma (irrespective of glioma grade). The mean value of ODI was significantly
higher in high grade glioma (HGG) than in CNBA while ODI mean value was
significantly lower in low grade glioma (LGG). Compared with LGG, in
TP area, the min, mean, max values of ficvf, ODI and their ratios of TP/CNBA
are significantly higher in HGG (p<0.007) and the mean value of ficvf (0.40HGG
vs. 0.23LGG) showed the highest diagnostic value (AUC=0.81, cut-off
value: 0.33) and specificity (82%). Further, in PT area, the max values of
ficvf and ODI and the mean value of ODI are significantly higher in HGG
(p<0.038) and the max value of ficvf (0.48HGG vs. 0.36LGG)
demonstrated the highest diagnostic value (AUC=0.62) with the highest
specificity (87%) and relatively lower sensitivity (44%).
Dissusion: Glioma is a kind of neoplasm and characterized by
varying degrees of hypercellularity, nuclear pleomorphism, endothelial
proliferation and microvascular density. All those transforms in replace of the
normal brain tissue could modify the brain mircroenviroment, hence, would
change the freedom of water molecules to diffuse within the tissue. We found that
the ficvf and odi were significantly higher in HGG than in LGG. The higher
tumor cellularity, microvascular desity and much complicated endothelial
proliferation of HGG8,9 might cause those differences. The higher
tumor cellularity would accompany with higher axon density, therefore, the ficvf
was higher. Since the more restriction by the tumor cell, to some extent, might
induce higher index of orientation dispersion. The normal brain tissue with
blood brain barrier (BBB) usually have higher ficvf, therefore our study showed
that, irrespective of glioma grades, the ficvf was lower in glioma. However,
for ODI, the ODI in HGG was higher than in norm brain tissue. This might due to
the higher tumor cellularity and the serious destroy of the BBB and the LGG
with relatively less tumor cellularity and less BBB destroy, therefore, the ODI
was significantly lower than in normal brain. For PT area, compared with LGG,
the signal abnormality in HGG is not only caused by the altered interstitial water
but also by the infiltration of the scattered tumor cells10,11and this
might cause the differences in ficvfmax、odimean、odimax.
Conclusions: Quantitative
parameters from NODDI in TP and PT area can aid in gliomas grading and the mean
value of ficvf showed the highest diagnostic power.
Acknowledgements
No acknowledgement found.References
1. Sadeghi N, D'Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies[J]. AJNR Am J Neuroradiol,2008,29(3):476-482. 2. Calvar J A, Meli F J, Romero C, et al. Characterization of brain tumors by MRS, DWI and Ki-67 labeling index[J]. J Neurooncol,2005,72(3):273-280. 3. Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging[J]. Neuroradiology,2007,49(10):795-803. 4. Hui Z, Torben S, Claudia A, et al. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61 (2012) 1000–1016. 5. Magnollay L, Grussu F, Wheelerkingshott C,et al. An investigation of brain neurite density and dispersion in multiple sclerosis using single shell diffusion imaging. In: (Proceedings) Joint Annual Meeting ISMRM-ESMRMB. (pp. 2048). 6.Winston G, Micallef C, Symms M, et al. Advanced diffusion imaging sequences could aid assessing patients with focal cortical dysplasia and epilepsy. Epilepsy Res. 2014 Feb;108(2):336. 7. Zaja-Milatovic S, Milatovic D, Schantz AM, et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64(3):545547. 8. Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001; 22:1081–88. PMID: 11415902 Synopsis 9.Toh CH, Wei KC, Ng SH, et al. Differentiation of tumefaetive demyelinating lesions from high-grade gliomas with the use of diffusion tensor imaging [J]. AJNR, 2012, 33(5): 846-851. 10. Burger P. Classification, grading, and patterns of spread of malignant gliomas. In: Apuzzo ML, ed. Neurosurgical topics: malignant cerebral glioma. Park Ridge, Ill: American Association of Neurological Surgeons.1990;3–17. 11. Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma: pathologic criteria and prognostic implications. Cancer. 1985; 56: 1106–1111.Figures
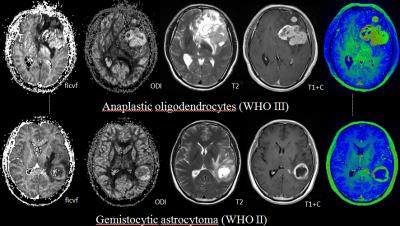
There were two cases with left frontal lobe
brain tumor (upper) and left temporal lobe (below). Both cases showed vivid
enhancement and serious necrosis. The ficvf and ODI was higher in the upper
case than the lower one. The pathology showed that upper tumor was a higher
grade glioma while the lower one was lower grade glioma.