4174
True progression versus radiation necrosis in glioma: a comparative study of arterial spin labelling and dynamic susceptibility contrast imaging1Department of radiology, Affiliation hospital of Xuzhou Medical University Department of Radiology, Xuzhou, People's Republic of China, 2GE Healthcare, Shanghai, People's Republic of China
Synopsis
Differentiation of treatment-related radiation necrosis from recurrent neoplasm is often difficult and dynamic susceptibility contrast perfusion MR imaging (DSC-MRI) is reported to be a surrogate marker for distinguishing them. However, DSC technique is invasive and has its disadvantage. Three dimensional pseudo-continuous ASL (3D-pcASL) can provide noninvasive absolute cerebral blood flow (CBF) measurement with insensitivity to permeability, and it is especially necessary for the evaluation of the postoperative gliomas patients where the blood-brain barrier (BBB) is completely broken with much more leakage effects. This study aimed to differentiate true progression from radiation necrosis of gliomas by using 3D-pcASL and DSC-MRI.
Purpose
Gliomas represent approximately 27% of all tumors and 80% of malignant tumors1. Most patients with gliomas undergo surgical resection followed by concurrent chemoradiotherapy (CCRT). Neoplastic recurrence is common and early detection of recurrence is crucial in patient care. Differentiation of radiation necrosis from recurrent neoplasm is often difficult with conventional magnetic resonance (MR) imaging because both entities share common features such as enhancement, mass effect, and vasogenic edema2. Dynamic susceptibility contrast perfusion MR imaging (DSC-MRI) is a surrogate marker for angiogenesis and has been used to assess brain tumor treatment response with high sensitivity for distinguishing recurrent glioma from radiation brain injury3. However, DSC-MRI is based on the injection of the contrast agent and the assumption that the gadolinium-based contrast agent (GBCA) remains within intravascular space, and violation of this could cause leakage effects, which is common in postoperative brain glioma and confounds rCBV and rCBF measurements. Three dimensional arterial spin labeling (3D-ASL) is a noninvasive magnetic resonance perfusion imaging technique to measure cerebral blood flow (CBF), by using magnetically labeled arterial blood water as an endogenous contrast medium4. Previous studies5,6 mainly focused on its application on the evaluation of primary brain tumors and ischemic stroke, researches about the differentiation of radiation necrosis from recurrent neoplasm of postoperative gliomas were few. The purpose of this study was to differentiate true progression from radiation necrosis of gliomas in postoperative-glioma patients treated with CCRT by using 3D-pcASL and DSC-MRI.Methods
Twenty-six consecutive patients who showed new or enlarged, contrast-enhancing lesions within the radiation field after CCRT were assessed by use of 3D-ASL and DSC-MRI. DSC-MRI was performed using a T2*-weighted single-shot gradient-recalled echo-planar imaging (GRE EPI) sequence, and ASL perfusion imaging was performed with pseudo-continuous labelling, background suppression and a stack of spirals 3D fast spin echo imaging sequence. These patients were classified into groups of tumor recurrence (n=16) or radiation necrosis (n=10) based on pathologic analysis or clinical-radiologic follow-up. The extent of susceptibility artefacts in the enhanced lesions was scored from 1 to 3 (1 = no susceptibility artefacts and 3 = extensive susceptibility artefacts (maximum diameter>2 cm))6. A quantitative analysis was performed with cerebral blood flow values (ASL-CBF), relative cerebral blood flow values (ASL-rCBF, DSC-rCBF) and relative cerebral blood volume values(DSC-rCBV) in the region of interest (ROI) with maximum signal enhancement. In addition, receiver operative characteristic (ROC) curves were performed to define thresholds of ASL-CBF, ASL-rCBF, DSC-rCBF, DSC-rCBV values that could best predict tumor recurrence.Results
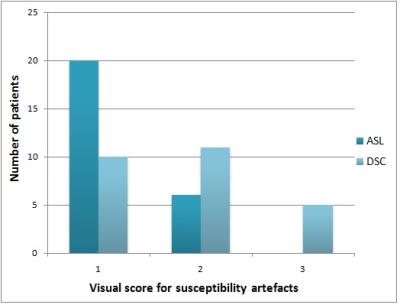
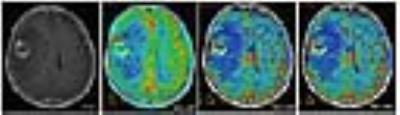
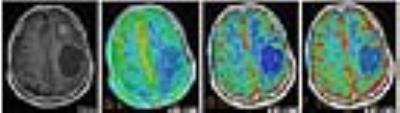
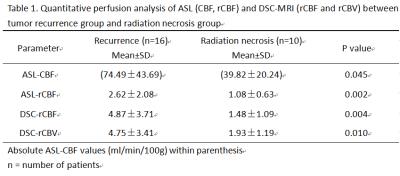
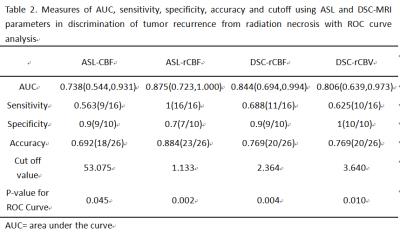
ASL had a lower susceptibility-artefact score than DSC-MRI (p=0.01) (Fig. 1). On a Bland-Altman plot, differences for ASL-rCBF and DSC-rCBF ASL values were on average -1.54±2.87 (mean ± SD) with limits of agreement 4.1 -7.2, bias -1.5. There was a statistically significant difference between tumor recurrence group and radiation necrosis group for all parameters (Table 1, Fig. 2, Fig. 3). There was good correlation between DSC-rCBF and ASL-rCBF values, as well as DSC-rCBV and ASL-rCBF values with correlation coefficients of 0.8 and 0.727. On the basis of the quantitative analysis, receiver-operating characteristic (ROC) curves were constructed to define the optimal cutoff value; the highest sensitivity value for ASL-rCBF was obtained using a cutoff value of 1.133, resulting in sensitivity of 100% and specificity of 70% (Table 2).Discussion
We found that ASL CBF images had significantly fewer susceptibility artefacts than did DSC-MRI maps. Tumors situated close to bone-air interfaces are particularly vulnerable to susceptibility artefacts. Spiral acquisition and FSE instead of single-shot GRE EPI explain the low number of susceptibility artefacts seen in ASL compared with DSC GRE EPI maps in our study7. Most previous MRI studies have used DSC rCBV for the evaluation of brain tumor perfusion10,11. This study, in which ASL-rCBF values correlated with the corresponding DSC-rCBV values (r=0.727) and DSC-rCBF values (r=0.80), as well as good agreement between ASL-rCBF and DSC-rCBF, confirms that ASL may be as good as DSC for the assessment of tumor angiogenesis. In our study, quantitative analyses of ASL-rCBF provided the highest sensitivity compared to DSC-MRI for differentiating radiation necrosis from glioma recurrence using a cutoff value of 1.133 without false-negative result. The low sensitivity of DSC-MRI findings likely arose from vascular leakage, probably caused by radiation necrosis8. Other possible reasons are intratumoral susceptibility artifacts due to recent hemorrhage or chronic hemosiderin deposition9.Conclusion
3D-pcASL is superior than DSC-MRI for the evaluation of perfusion in differentiating true progression from radiation necrosis of gliomas treated with CCRT. This method is invasive, and has fewer susceptibility artefacts and higher sensitivity than DSC-MRI.Acknowledgements
No acknowledgement found.References
1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17:iv1-iv62.
2. Mullins ME, Barest GD, Schaefer PW, et al. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26(8):1967-1972.
3. Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575-584.
4. Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol. 2010,2(10): 384-398.
5. Huang YC, Liu HL, Lee JD, et al. Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MRI in patients with acute stroke. PLoS One. 2013,8(7):e69085.
6. Järnum H, Steffensen EG, Knutsson L, et al. Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology. 2010;52(4):307-317.
7. Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48(2):242-254.
8. Balentova S, Adamkov M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int J Mol Sci. 2015;16(11):27796-27815.
9. Bagley LJ, Grossman RI, Judy KD, et al. Gliomas: correlation of magnetic susceptibility artifact with histologic grade. Radiology 1997; 202(2):511–516.
10. Zhang H, Rödiger LA, Shen T, et al. Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology. 2008;50(10):835-840.
11. Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006;48(10):703-713.
Figures