4171
Proposed thresholds for parametric response mapping using intravoxel incoherent motion diffusion weighted imaging (IVIM-DWI) for the assessment of brain tumor treatment response1Translational Bioimaging Group, Barrow Neurological Institute, Phoenix, AZ, United States, 2Imaging Programs, National Comprehensive Cancer Network (NCCN), Philadelphia, PA, United States, 3University of Texas - Austin
Synopsis
The purpose of this study is to establish thresholds for parametric response mapping (PRM) using apparent diffusion coefficients (ADC) and intra-voxel incoherent motion (IVIM) parameters in healthy controls and to apply these thresholds in a cohort of brain tumor patients to study regional treatment-induced changes in diffusion and perfusion. We obtained thresholds (95% confidence intervals) of 0.38 and 0.32 x10-3 mm2/s for ADC and IVIM-D, respectively. For the perfusion-related distributions, the thresholds were 10 ml/100g (IVIM-fp) and 54 ml/100g/min (IVIM-fpD*). This multi-parametric sensitivity to local tumor changes could be useful to simultaneously evaluate treatment-induced changes in perfusion and cellularity.
Introduction
Parametric response mapping (PRM) is a voxel-wise approach to assess regional heterogeneity of longitudinal MRI changes associated with treatment. PRM is most often applied to apparent diffusion coefficients (ADCs)1,2, and to a lesser extent rCBV3,4, and is predictive of long-term brain tumor treatment-induced effects.5 In the context of diffusion, the PRM thresholds reflect the 95% confidence intervals for normal-appearing tissue and can indicate tumor hypercellular (positive change) and hypocellular (negative change) status.6 Intra-voxel incoherent motion (IVIM) is increasingly used for oncologic imaging and informs on perfusion-insensitive diffusion and perfusion related metrics without contrast bolus injection.7 This multi-parametric sensitivity could be useful to simultaneously evaluate treatment-induced changes in perfusion and cellularity, as may be expected with successful anti-angiogenic treatment. The purpose of this study is (i) to establish thresholds for PRM using ADC and IVIM parameters in healthy controls and (ii) to apply these results in a cohort of brain tumor patients to study regional treatment-induced changes in diffusion and perfusion.Methods
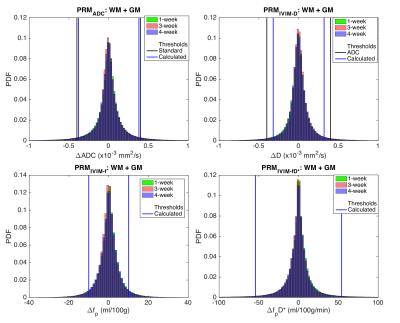
Data were acquired at 3T (Achieva, Philips Healthcare) in healthy controls (n = 9) and high-grade glioma patients (n = 6) at three time points (time 0, 1 week, and 4 weeks). The patient data corresponded with before and two time points after Bevacizumab treatment. Images were acquired with a single-shot spin-echo EPI sequence with TR = 6 s, TE = 50 ms, SENSE parallel imaging (2 in AP direction), voxel size = 2.5 mm isotropic, 40 slices, with three orthogonal diffusion-encoding directions and 8 b-values (b = 0, 25, 50, 75, 100, 200, 500, 1000 s/mm2). All data were registered to the T1-weighted image obtained at the 2nd time point using the affine (12 DOF) registration algorithm FLIRT (FSL, FMRIB Centre, Univ. of Oxford). The segmentation algorithm FAST (FSL) was used to segment white matter (WM) and gray matter (GM) regions of interest (ROIs). Tumor ROIs were drawn on the post-contrast T1-weighted image obtained pre-treatment (T0) and applied to all time points. The diffusion data from b = 0 and 1000 were fit to a mono-exponential model to obtain ADC. For IVIM, a multi-step fitting procedure was used; first, signals from b > 200 s/mm2 were fit to a mono-exponential model to obtain IVIM-D, subsequently, all b-values were fit to a constrained bi-exponential model to obtain IVIM-fp and IVIM-D*. IVIM data were converted to standard perfusion units using the appropriate conversion factors, where CBV is proportional to fp and CBF is proportional to fpD*.7 Using healthy control WM and GM combined ROIs with 1-week (T1-T0), 3-week (T3-T1), and 4-week (T3-T0) time intervals, the voxel-wise pooled standard deviation was calculated for each metric (ADC, IVIM-D, IVIM-fp, and IVIM-fpD*). From the pooled standard deviations, 95% confidence intervals (95% CIs) were obtained for each metric, and these 95% CIs were then applied to the brain tumor patient data.Results / Discussion
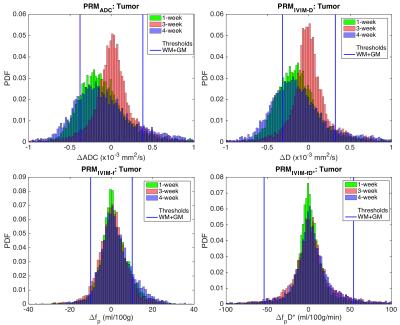
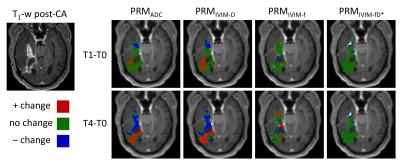
Figure 1 shows the pooled distributions of ADC and IVIM changes for healthy control WM+GM combined ROIs over 1-week (green), 3-week (red), and 4-week (blue) time intervals. Previous studies have determined and validated ΔADC thresholds using patient normal-appearing WM+GM ROIs, concluding that 0.40 x10-3 mm2/s is the optimized ADC threshold (shown as black vertical lines for ADC and IVIM-D).6 Similarly, we obtained thresholds of 0.38 and 0.32 x10-3 mm2/s for ADC and IVIM-D, respectively. For the perfusion-related distributions, the 95% CIs were 10 ml/100g (IVIM-fp) and 54 ml/100g/min (IVIM-fpD*). Applying these thresholds to the pooled tumor ROIs over all subjects (Figure 2) shows a wider distribution of treatment-induced changes, where voxels falling outside the thresholds may indicate significant treatment effects. The advantage of a voxel-wise method is that sensitivity to local tumor heterogeneity is preserved, unlike whole tumor mean changes that may miss regional treatment-induced changes. Figure 3 demonstrates the application of these PRM thresholds in a brain tumor patient over 1-week and 4-weeks post-treatment. The thresholds for ADC and IVIM-D are similar (0.38 and 0.32 x10-3 mm2/s, respectively), yielding similar PRMs with more voxels of significant change occurring for IVIM-D due to the narrower threshold. PRM for IVIM-fp (perfusion) shows a dissimilar pattern compared to diffusion, reflecting differences in their underlying sensitivity to diffusion and perfusion effects. The thresholds for IVIM-fpD* are much wider, indicating lower voxel-wise repeatability, and thus there were limited significant changes with treatment for this parameter.Conclusions
This study establishes thresholds for PRM with IVIM perfusion related metrics, and this method may separate local regions of tumor progression from treatment effects. Further investigation is ongoing into the relationship between PRM and patient outcomes.Acknowledgements
This work was supported by NIH/NCI 1R01CA158079, NIH/NCI U01 CA142565, NIH/NCATS KL2 TR 000446, and NIH/NCATS RR024975.References
1. Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proceedings of the National Academy of Sciences of the United States of America 2005;102(15):5524-5529.
2. Ellingson BM, Malkin MG, Rand SD, et al. Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas. Journal of neuro-oncology 2011;102(1):95-103.
3. Kickingereder P, Radbruch A, Burth S, et al. MR Perfusion–derived Hemodynamic Parametric Response Mapping of Bevacizumab Efficacy in Recurrent Glioblastoma. Radiology 2016;279(2):542-552.
4. Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nature medicine 2009;15(5):572-576.
5. Hamstra DA, Galban CJ, Meyer CR, et al. Functional Diffusion Map As an Early Imaging Biomarker for High-Grade Glioma: Correlation With Conventional Radiologic Response and Overall Survival. Journal of Clinical Oncology 2008;26(20):3387-3394.
6. Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. Journal of Magnetic Resonance Imaging 2010;31(3):538-548.
7. Bihan DL, Turner R. The capillary network: a link between ivim and classical perfusion. Magn Reson Med 1992;27(1):171-178.
Figures