4165
Diffusion magnetic resonance imaging for predicting changes in the severity of Progressive Supranuclear Palsy1Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan City, Taiwan, 2Medical Imaging and Intervention, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 3Neurology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan
Synopsis
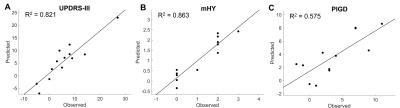
Progressive Supranuclear Palsy (PSP) is an atypical Parkinsonism, which shared similar symptoms with Parkinson’s disease (PD) and PSP progressed typically much faster than PD and the prognosis is often poor. The linear regression analysis demonstrated the capability of diffusion MRI indices as measured from multiple brain regions in the prediction of two-year clinical severity. Strong predictive power can be observed in mHY, motor subscale of UPDRS and PIGD. The two-year clinical decay in patients with PSP can be accurately predicted by using diffusion tensor derived parameters as measured from distinct brain regions.
PURPOSE
Progressive Supranuclear Palsy (PSP) is an atypical Parkinsonism, which shared similar symptoms with Parkinson’s disease (PD) such as bradykinesia, blepharospasm, and dysarthria1. The most characteristic symptom PSP is supranuclear ophthalmoplegia, a limitation of eyeball movement in vertical direction2. Unfortunately, PSP progressed typically much faster than PD and the prognosis is often poor. Because of the involvement of cortical syndromes, a more general neural network might be disturbed. The hypothesis is that the microenvironmental changes from multiple brain regions, as detected by diffusion MRI, could reflect the disease severity. Therefore, the current study proposed to examine the prognostic performance of the combination of multiple diffusion tensor derived indices to predict patient’s clinical outcome in a longitudinal study.METHODS
The study was approved by the Institutional Review Board. Diffusion tensor images were acquired from 13 PSP patients (aged 62.7±4.9 year old) from a 3T MR scanner (MAGNETOM Trio a TIM system, Siemens, Germany). The imaging parameters from the EPI sequence are TR/TE = 5100ms/91ms, voxel size=2*2*2 mm, 64 directions, b value = 0 /1000/ 2000 sec/mm2. T1-weighted images were acquired using a magnetization-prepared rapid acquisition gradient echo sequence (TR/TE = 1700ms/2.63ms, flip angle = 9°, voxel size= isotropic 1 mm). The diffusion images was parcellated into 116 brain regions according to the Automatic Anatomical Labeling follow by the procedure by refer our paper in european radiology by Lu et al3. The 90th, 50th, and 10th percentiles of the diffusion parameters (Mean/Axial/Radial Diffusivity and Fractional Anisotropy) were calculated. Pearson correlations were used to get the correlation between baseline diffusion parameters and the difference in clinical graded. Clinical assessments were graded from two time points (baseline and after a mean of 828.3±291.8 days), including Unified Parkinson’s Disease Rating Scale (UPDRS), Modified Hoehn and Yahr (mHY) and Postural Impairment and Gait Disorder (PIGD). Pearson’s correlation was performed between diffusion indices in AAL regions and the difference of clinical scores. A linear regression was performed with selected brain regions with significant correlation to estimate the prediction formula of clinical grades4. Two-tailed p values<0.05 corrected for multiple correction were considered statistically significant.RESULTS
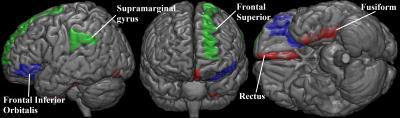
Regression analysis between selected brain regions and clinical assessments was performed in Table 1. The highest predictive power (adjusted R2) of diffusion indices was mHY (R2 = 0.86). In addition, the predictive values in motor subscale of UPDRS and PIGD were 0.821 and 0.575, respectively. The predicted and observed scores for the motor subscale of UPDRS, mHY, and PIGD agree satisfactorily (Figure 1). Figure 2 is a three-dimensional volume-rendered image of the brain regions which involved in the regression analysis of different clinical assessments. The involved brain regions were located in fusiform and rectus (motor subscale of UPDRS, in red), superior Frontal and supramarginal gyrus (mHY, in green), and orbital part of inferior frontal lobe (PIGD, in blue).DISCUSSION
The linear regression analysis demonstrated the capability of DTI indices as measured from multiple brain regions in the prediction of two years clinical severity. Strong predictive power can be observed in mHY, motor subscale of UPDRS and PIGD. This finding support the hypothesis that the DTI indices could potentially be predictive of the patients’ disease progression in the future. Noticeably the prediction is made from RD and FA, which might suggest an increased sensitivity than AD and MD5. The functions of the affected brain regions are consistently related to the changed motor performance in PSP patients.CONCLUSION
The two-year clinical decay in individuals with progressive supranuclear palsy can be accurately predicted by using diffusion tensor derived parameters as measured from distinct brain regions.Acknowledgements
No acknowledgement found.References
1. Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. American journal of epidemiology. 2003;157:1015-22.
2. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1-9.
3. Lu CS, Ng SH, Weng YH, et al. Alterations of diffusion tensor MRI parameters in the brains of patients with Parkinson's disease compared with normal brains: possible diagnostic use. European radiology. 2016;26:3978-88.
4. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41:1149-60.
5. Chand P, Litvan I et al, Neurobiology of progressive supranuclear palsy-9, Elsevier Inc. 2007, Neurobiology of Disease Pages 105–110DOI: 10.1016/b978-012088592-3/50011-6.