4150
Abnormal Brain White Matter Connectivity in Diabetic Neuropathy: A Magnetic Resonance Diffusion Tensor Imaging Study.1University of Sheffield, Sheffield, United Kingdom, 2Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
Synopsis
This study for the first time has shown alterations in white matter MD in patients with DSPN. Increased MD in the primary somatosensory cortex in patients with diabetic neuropathy (DN) is suggestive of white matter microarchitecture degeneration, and supports the evidence of neuronal loss in the somatosensory cortex in patients with . Furthermore, these results also support the previous evidence of thalamic neuronal dysfunction in DN on MR spectroscopy. Changes in the degree of white matter structure might provide a pathophysiological underpinning of spinal cord atrophy and brain volume reduction in DN.
Introduction
Diabetes is estimated to affect 415 million people worldwide and worryingly its prevalence is projected to increase significantly, affecting 642 million adults by 2040 (1) . Painful diabetic neuropathy (DN) is a serious complication affecting up to 20-26% of these patients (2,3). With the increasing prevalence of diabetes, DN will pose a major treatment challenge (4,5). Painful DN causes burning, deep aching, “electric shock” like, lancinating (also likened as “stabbing or knife like” pains) in the extremities. Moderate-to-severe unremitting lower limb pain is present in over 70% of sufferers (3,6) and causes insomnia, poor QoL, unemployment, and depression (7-10). Unfortunately, there are no effective ways of treating painful DN. The risk factors are not known and can’t be addressed to improve prognosis. The pathophysiology of painful DN remains poorly understood (5) and there are no agreed disease modifying treatments. We have previously demonstrated significant brain and spinal cord volume loss in DN. This study aims to investigate integrity of cerebral white matter tracts using MR Diffusion Tensor Imaging (DTI).Methods
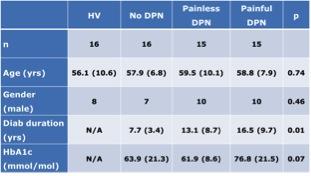
Forty-six patients with diabetes and 16 healthy volunteers [mean age 56.1+ SD 10.6 years] underwent DTI. Each of the subjects also underwent detailed established clinical/neurophysiological assessments (including quantitative sensory testing, autonomic function tests and nerve conduction studies) to quantify the severity of DN. Based on a composite DN score, subjects with diabetes were divided into three groups: 16 without DN (No-DN [7, 57.9+ 6.8]); 15 subjects with painless DN (10, 59.5+10.1) and 15 with painful DN (10, 58.8+7.9][IW1] Imaging was performed at 3T (Ingenia 3.0T, Philips Healthcare, Best, The Netherlands) using a receive-only 32 channel d-stream head coil. The imaging protocol included high angular resolution DTI (32-directions, b=0, 1000 s/mm2, voxel size=1.75×1.75×2.0mm) based on a 2D-EPI readout sequence. Mean fractional anisotropy (FA) and mean diffusivity (MD) maps were generated for each group using the FMRIB Software Library (FMRIB, Oxford, UK) Tract-Based Spatial Statistics (TBSS) analysis program and compared using FSL view (results cluster corrected, p<0.05). [IW1]Can’t see what the ’10’ is for - is that the actual number from a previous abstract with a mean age of the following number ?Results
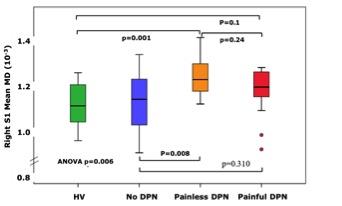
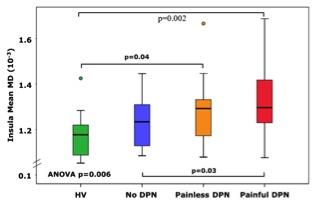
Study groups were matched for age, gender and glycaemic control (Figure 1). Subjects with No-DN had significantly lower duration of diabetes compared to painful and painless DN groups. Voxelwise subgroup analysis showed significantly greater MD in subjects with painful and painless DN compared to healthy volunteers in somatosensory tracts (p = 0.01 and 0.03 respectively). There was greater disruption in white matter track integrity in painless DN in somatosensory regions (S1, HV 1.10+ 0.09, No DN 1.10+0.13), painless DN 1.23+0.09 painful DN 1.17+0.11; ANOVA P=0.006, Figure 2). Painful DN subjects had greater disruption in affective nociceptive processing regions (insula, HV 1.18+0.1o, No DN 1.23+0.10, painless DN 1.27+0.15 and painful DN 1.19+0.12; ANOVA p=0.006, Figure 3). There were no brain regions where MD was higher in HV or No-DN subjects compared to DN.Conclusions
This study for the first time demonstrates significant white matter alterations in DN. There was greater disruption within somatosensory regions in painless DN. Whereas, in painful DN affective pain processing regions most affected. This may provide clues to the pathogenesis of different sensory phenotypes of DN. Furthermore, our findings build on past research providing a pathophysiological underpinning of spinal cord atrophy and brain volume reduction in DN.Acknowledgements
No acknowledgement found.References
1. International Diabetes Federation (2016). Diabetes Atlas (International Diabetes Federation).
2. Abbott C, Malik R, Van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–4.
3. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22.
4. Veves A, Backonja M, Malik R. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–74.
5. Boulton A, Vinik A, Arezzo J. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62.
6. Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47(2):123–8.
7. Zelman DC, Brandenburg N, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain. 2006;22(8):681–5.
8. Gore M, Brandenburg N, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity indiabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374–85.
9. Gore M, Brandenburg N, Hoffman D, Tai K-S, Stacey B. Burden of Illness in Painful Diabetic Peripheral Neuropathy: The Patients’ Perspectives. J Pain. 2006;7(12):892–900.
10. Tolle T, Xu X, Sadosky AB. Painful diabetic neuropathy: A cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006;20(1):26–33.