4140
Development of the oligomeric amyloid-beta targeted MRI contrast agents to diagnose the early stage of Alzheimer’s diseaseGeon-Ho Jahng1, Sang-Tae Kim2, Peter Verwilst3, Hyug-Gi Kim4, Jee-Hyun Cho5, Kwan Soo Hong5, Ki Woong Kim2, Jong Seung Kim3, Wook Jin1, Eui Jong Kim6, and Dal Mo Yang1
1Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea, Republic of, 2Bundang Hospital of Seoul National University, Kyunggeedo, Korea, Republic of, 3Korea University, Seoul, Korea, Republic of, 4Kyung Hee University, Seoul, Korea, Republic of, 5Korea Basic Science Institute, Cheongju, Korea, Republic of, 6Radiology, Kyung Hee University Hospital, Seoul, Korea, Republic of
Synopsis
A new T1 molecular MRI contrast agent specifically designed to be specific for oligomeric Aβ was developed by combining the commercially available gadolinium (Gd)-Dota with an oligomeric Aβ-specific DNA aptamer. We confirmed the protein size with Aβ polymerization in aspect of molecular masses when polymers were formed. We performed the following experiment in the cell level and AD-model mice. We identified a particular region with a significantly distinct T1 MRI signal, allowing for distinguishing Alzheimer's disease model mice from non-Tg mice.
Background and Objective
One of the peptides showing an aberrant distribution in Alzheimer’s disease (AD) patients is amyloid-beta (Aβ) and is cleaved from the C-terminal fragment of the amyloid precursor protein (APP) in the cortex and hippocampus. The oligomeric forms of the aberrant tau and APP cleavage products play a crucial role in the early stages of AD, when cognitive impairments may be seen in the absence of obvious neuronal loss or feature formation (1,2). Therefore, in order to facilitate early stage diagnosis in AD patients, it is pivotal to target oligomeric Aβ rather than amyloid plaques. Radioisotopes using positron emission tomography (PET) are recently being applied in humans to visualize amyloid deposits in AD, mild cognitive impairment (MCI) and healthy aged individuals (3). However, PET imaging is limited to repeated scans of elderly subjects to early detection of oligomeric Aβ types or amyloid plaques because of exposure of radiation in the human body. In addition, the low spatial resolution of PET does not allow the imaging of small plaques and it is not clear if it will be able to visualize the earliest stages of amyloid pathologies. Therefore, the objective of this study was to develop a novel MRI contrast agent to target the oligomer Aβ, allowing for the in vivo determination of the early forms of the AD pathology by monitoring the signal changes after intravenous injection of the contrast agent.Materials and Methods
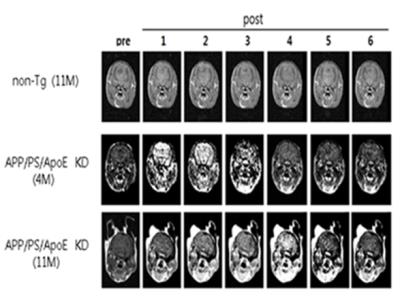
The new contrast agent was developed by combining the commercially available gadolinium (Gd)-Dota with an oligomeric Aβ-specific DNA aptamer (called as oAB). The aim of conjugating to aptamer was not only to increase the targeting ability vs. the oligomeric forms of beta amyloid but also to increase the conjugates’ BBB penetrability. This new agent was called as Gd-oAB. We confirmed the protein size with Aβ polymerization in aspect of molecular masses when polymers were formed. We performed the following experiment in the cell level and AD-model mice. First, to determine the technical feasibility of optimal quenching, the oAb was combined with QD525-BHQ1 beacon. The quenching efficiently was examined by incubating fixed concentration of the QD525-oAB-BHQ1 nanoparticle (100 pmol) without or with 1μM oAβ in a mouse hippocampal HT22 cells at 37℃ for 12h. Confocal microscopy imaging with oAβ observed significantly green fluorescence signals of HT22 cells after 12h of oAβ treatment. Second, to determine the high affinity and selectivity of the new contrast agent, Gd-oAB-cy5 was used to evaluate brain-blood barrier (BBB) permeability. Cultures of mouse cells were incubated with different oAβ concentration and probed with QD525-oAB and imaged using a fluorescence dye. We determined if Gd-ob5-cy5 modulates the cellular protein clathrin H/T, caveolin and transferrin receptor which control oAβ endocytosis in mouse cerebral cortex bEND3 and mouse hippocampal HT22 cells. Third, to acquire in vivo MR images, all MRI experiments were performed on a 4.7 T animal MRI scanner (BioSpec 47/40; Bruker, Ettlingen, Germany) with respiratory gating (SA Instruments, Stony Brook, NY, USA). To investigate the signal behaviors in the brain after injection of the new contrast agent, T1-weighted(T1W) images were acquired with multiple times before (pre) and after (post) injection of the Gd-oAB contrast agent obtained from non-Tg (11M old mice, old normal), APP/PS/ApoE KD (4month-old, young AD), and APP/PS/ApoE KD (11M old mice, old AD).Results
First experiment: Confocal microscopy imaging with oAB showed that Rod-shaped QD-oAB particles were homogenously dispersed in an aqueous solution, and the hydrodynamic particle size measured using transmission electron microscopy (TEM) was about 24.5nm. These results demonstrated that the technical feasibility of fluorescent Gd-oAB or Gd-oAB-cy5 probe for early stage in Alzheimer’s disease pathogenesis could successfully provide images of the intracellular or extracellular oAβ with great specificity. Second experiment: Gd-oAB-cy5 probe-treated bEND3 highly expressed caveolin1 and transferrin receptor but HT22 cells highly expressed almost gate-mediated endocytosis. These results supports that Gd-oAB-cy5 may be stimulated caveolin1 and transferrin receptor expression in related with BBB permeability, and that Gd-oAB-cy5 plays an important role to bind oAβ behavior via multiple endocytotic pathways near BBB. Third experiment: In the non-Tg mouse, signals in the brain after injection of the new contrast agent were almost similar to those of before injection of the contrast agent. However, in the young (4 month-old) and old (11month-old) APP/PS/ApoE KD mice, signals in the brain were increased after injection of the new contrast agent. We found early increase of signals in the young KD mouse which has developed oAβ in the brain, but delayed increase of signals in the old KD mouse which has developed Aβ plaque in the brain.Conclusion
Current research points towards oligomeric Aβ as an early-onset hallmark that provokes irreversible neuronal loss and progressive cognitive decline. Here, we report a discrete T1 molecular MRI contrast agent specifically designed to be specific for oligomeric Aβ as well as amyloid plaques. The complex, comprising an Aβ-specific aptamer conjugated to a MRI contrast agent is selective and exhibits a high affinity to oligomeric Aβ both on cells and brain tissues. We identified a particular region with a significantly distinct T1 MRI signal, allowing for distinguishing Alzheimer's disease model mice from non-Tg mice. Using this new contrast agent, selectively targeting neurotoxic Aβ oligomers could be potentially useful for validating the efficacy of novel diagnostic agents and could ultimately predict early-stage onset Alzheimer's disease prognosis and pave the way for disease control.Acknowledgements
This work was supported by the Juam as well as grants from the YJK Foundation (Seoul, Korea). We thank L. Hyun Seung for support with mouse work and L. Soo Hwan for support with bEND3 cells. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP); contract grant number 2014R1A2A2A01002728.References
1. Lambert MP, et al (1998) Proc Natl Acad Sci USA 95:6448-6453;
2. Walsh DM, et al (2002) Nature 416:535-5539.
3. Ono M and Saji H (2015) Med Chem Commun 6:391–402.