4137
Automated Fiber Quantification Identifies the Extent of White Matter Integrity Loss Concurrent to Hippocampal Atrophy1Radiology, Medical University of South Carolina, Charleston, SC, United States, 2Neurology, Medical University of South Carolina, Charleston, SC, United States, 3Neuroscience, Medical University of South Carolina, Charleston, SC, United States
Synopsis
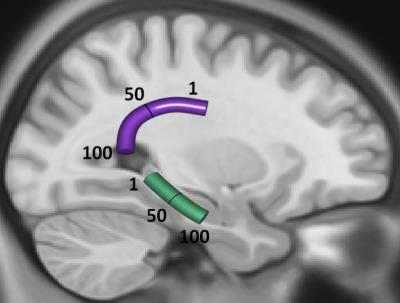
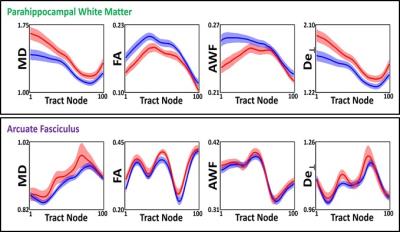
Automated fiber quantification with white matter tract integrity metrics identified subtle changes in brain microstructure occurring in the early stages of Alzheimer’s pathology. These metrics were found to be significantly different in the posterior section of the parahippocampal white matter in cognitively normal older adults with hippocampal atrophy as compared to those without. Axonal water fraction distinguished those with hippocampal atrophy versus those without with the largest effect size (Cohen’s d = 0.86, p = 0.004). As expected, the metrics for these two groups did not differ in the arcuate fasciculus, a tract typically unaffected in Alzheimer’s disease.
INTRODUCTION
Automated fiber quantification (AFQ), a novel tractography-based image analysis technique1, used in conjunction with white matter tract integrity (WMTI) metrics2 is a promising method for identifying subtle loss of white matter (WM) integrity due to aging or early stages of disease. Here, we investigate the utility of AFQ in conjunction with WMTI in differentiating cognitively normal older adults with and without hippocampal atrophy, as this could provide non-invasive imaging biomarkers of changes in tissue microstructure that occur early in the course of Alzheimer’s disease (AD), thus improving detection of disease and its progression.METHODS
MR images were obtained with a 3T Siemens TIM Trio. MPRAGE and FLAIR images were acquired for anatomical and WM hyperintensity assessment. Diffusional kurtosis images (DKI)3 were acquired using a twice-refocused spin-echo EPI diffusion sequence with b-values=0,1000,2000 s/mm2 with 64 homogeneously distributed gradient directions for each b-value and with 24 additional b=0 images. Diffusion parametric maps were calculated with Diffusional Kurtosis Estimator (DKE) software [http://www.nitrc.org/projects/dke]. WMTI metrics were computed as previously described2,3 and included axonal water fraction (AWF) and axial and radial extra-axonal water diffusivity (De,ax and De,rad). DKI data were incorporated into the AFQ image processing pipeline (https://github.com/jyeatman/AFQ) using fully automated in-house MATLAB scripts4.
AFQ utilizes diffusion tractography data and performs a series of automated steps to identify and segment specific WM fiber bundles and isolate the core of each tract1,5. Fiber bundles are selected by specifying regions of interest (ROIs), chosen from a WM template, which are applied to define the start and end points of each tract. Once the core of a tract is identified, AFQ interpolates a fixed number of nodes along the tract and estimates the diffusion and kurtosis tensors at every node, enabling reconstruction of all tensor-derived metrics4.
Twenty seven cognitively healthy NC (age=70.59±8.26; 19F) were studied. We examined parahippocampal WM (Figure 1A), as it includes axons that relay information to the hippocampus and is critical for memory formation. We contrasted these results with the arcuate fasciculus (Figure 1A), as this tract is typically unaffected early in the course of AD. To facilitate statistical testing of differences in tract nodes between the two groups, the tract nodes in Figure 1A were averaged into five bins.
RESULTS
NC subjects with (n=12) and without (n=15) hippocampal atrophy did not differ in demographic characteristics or neuropsychological testing. Significant changes in WMTI metrics were found in the posterior section of the parahippocampal WM in cognitively normal older adults with hippocampal atrophy versus those without, across several diffusion and WMTI parameters (Figure 1B, top row). Of all these parameters, AWF distinguished the two groups with the largest effect size (Tract node bin 1, Cohen’s d = 0.86, p = 0.004). As expected, these groups did not differ in their diffusion/WMTI parameters in the arcuate fasciculus (Figure 1B, bottom row).DISCUSSION
AD has a pathological cascade that long precedes its clinical manifestations. Disease-modifying therapies that address β-amyloid aggregation have thus far been relatively ineffective in ameliorating cognitive decline in the later stages of disease6. While amyloidosis is undoubtedly implicated in AD, we hypothesize that the earliest stages of AD consist of complementary pathological changes in WM on which alternative therapies and diagnostic tests can effectively focus. Indeed, WMTI metrics have previously been shown to be relevant for the assessment of AD2.
AFQ offers several advantages in circumventing the limitations of co-registration and manual tracings by using tractography to identify tracts within each individual and then quantifying the diffusion properties of equidistant nodes along these tracts. Additionally, AFQ is valuable in populations with widely varying brain volumes (as in aging and AD), where disease can significantly affect key sections but not the entirety of WM tracts. The results presented here, that were obtained utilizing a combination of AFQ with WMTI metrics, support the idea that loss of WM integrity may exist even prior to clinically manifest AD, and that WMTI metrics are potentially highly sensitive to detecting these subtle changes at the earliest stages of disease.
CONCLUSION
Although there is no clear consensus on the temporal order of brain microstructural changes that occur with AD7, diffusion-based MRI metrics remain viable biomarkers of AD8. Here we demonstrate that such metrics when used in combination with AFQ may provide useful information in the preclinical stages of AD and may provide evidence implicating the degeneration of WM in the earliest stages of AD9,10. This provides a strong rationale for developing biomarkers to detect WM changes in the pathogenesis of AD.Acknowledgements
Supported in part by NIH grant R01-AG027852 and The Litwin foundation.References
1) Yeatman JD, Dougherty RF, Myall NJ, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PloS one. 2012;7(11):e49790.
2) Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. NeuroImage. 2011;58(1):177-88.
3) Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698-7
4) Glenn GR, Jensen JH, Helpern JA, Spampinato MV, Kuzniecky R, Keller SS, Bonilha L. Epilepsy-related cytoarchitectonic abnormalities along white matter pathways. J Neurol Neurosurg Psychiatry. 2016 Sep;87(9):930-6.
5) Yeatman JD, Wandell BA and Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nature communications. 2014;5:4932.
6) Lemere CA & Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nature reviews Neurology. 2010;6(2):108-19.
7) Sexton CE, Kalu UG, Filippini N, et al. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiology of aging. 2011;32(12):2322.e5-18.
8) Schuff N. A new sensitive MRI marker for memory deficits in normal aging. Neurology. 2010;74(3):188-9.
9) Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dementia and geriatric cognitive disorders. 1998;9 Suppl 1:6-12.
10) Bronge L, Bogdanovic N and Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dementia and geriatric cognitive disorders. 2002;13(4):205-12.
Figures