4133
3D DCE-MR imaging shows compromised brain waste transport in spontaneously hypertensive rats1Center for Basic and Translational Neuroscience, University of Copenhagen, Copenhagen, Denmark, 2Department of Anesthesiology, Yale University, New Haven, CT, United States, 3Cellular and Metabolic Research & NMR, University of Copenhagen, Copenhagen, Denmark, 4Department of Neurosurgery, University of Rochester, Rochester, NY, United States
Synopsis
The link between hypertension and cerebral small vessel disease is key to understanding pathobiology of certain types of dementia. We studied the effects of mild hypertension on a newly discovered pathway for clearance of solutes from the brain parenchyma in young spontaneously hypertensive rats using DCE-MRI after intrathecal infusion of a paramagnetic contrast agent. We found normal-to-increased tracer influx and decreased efflux to and from the brain parenchyma, consistent with a lowered efficiency of brain solute clearance. This suggests that a compromised brain waste transport system may be implicated in the development of cerebral small vessel disease and dementia.
Introduction
Hypertension is a major risk factor for small vessel disease (SVD) of the brain causing dementia. Cerebral SVD is characterized by enlarged perivascular spaces and lacunes identifiable by clinical MRI as well as stiffening of the walls of penetrating arteries1,2. Although one of the most metabolically active body organs, the brain lacks a conventional lymphatic system for clearance of excess fluids and metabolites. Recently, our group discovered a fundamentally new pathway - the glymphatic system - consisting of periarterial cerebrospinal fluid (CSF) influx and perivenous efflux supported by convective bulk flow via astroglial AQP4 water channels, dedicated for transporting solutes such as amyloid-ß, tau and lactate out of the brain parenchyma [3]. Since SVD is often characterized by accumulation of excess fluid and structural remodeling of the perivascular space, we hypothesized that stagnation of glymphatic fluid transport plays a role in the pathogenesis of hypertensive cerebral SVD. We previously used dynamic contrast-enhanced (DCE) MRI to study glymphatic fluid fluxes, after administering paramagnetic contrast to the CSF of the cisterna magna (CM)4. Here, we use the same method to study the effect of mild hypertension on glymphatic transport function in spontaneously hypertensive rats (SHR) in comparison to normotensive Wistar Kyoto rats (WKY).Methods
Prior to imaging, a small glass cannula was implanted into CM and connected to a polyethylene tube for infusion of Gd-DOTA (Dotarem). Imaging was performed on a 9.4 T Bruker BioSpin magnet USR 94/30 using a volume transmit coil and a surface receive coil quadrature. Six 7-9 weeks old SHR and 7 age-matched WKY underwent successive spoiled gradient echo imaging (FLASH3D, TR: 15ms, TE: 4ms, FA: 15°, TA: 4min, FOV: 32x30x30mm3, Matrix: 128x128x128) before (NEX 3) and after (NEX ~40) onset of infusion of 13.5mM Gd-DOTA in saline at a rate of 1.5µL/min for a total volume of 20µL; total post-contrast scan time was approximately 150min. For surgery, anesthesia was induced using 2% Isoflurane and Ketamine (80mg/kg bodyweight), and maintained under 1-2% Isoflurane. During scanning, anesthesia was maintained using 0.6-0.8% Isoflurane and Dexmedetomedine (3-5µg/h/kg). Pulse oximetry, respiration, and temperature were monitored continually and kept within normal. Image processing included rigid motion correction, normalization to a 2mM Gd-DOTA phantom placed within the FOV during scanning, spatial normalization to a population specific template5. Then, percent change from baseline (%Δ) was computed, and ROIs were drawn in either template or native space, depending on structural homology. Care was taken in drawing ROIs to avoid spill-over from CSF in parenchymal signals.
Results
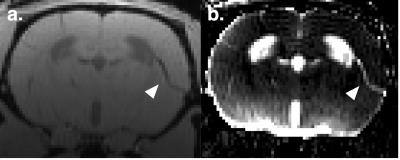
Time-contrast corves (TCCs) were extracted from key anatomical regions in brain and neck. In the striatum, cortex and the hippocamps TCCs showed accelerated glymphatic influx in SHR when compared to WT controls. (examples: Fig. 1b-e, although TCCs in olfactory bulb were very similar, Fig. 1e). Interestingly, the reverse was true for the cerebellum (Fig. 1f). Tracer clearance, measured as Gd-DOTA efflux along the carotid sheath was observed to be lower in SHR (Fig. 1g). As previously documented in the literature6 SHR rats were also characterized by enlarged ventricles compared to controls. We also observed rapid and prominent influx of contrast into the 3rd ventricle in SHR compared to WKY which may be attributable to differences in cardiac pulse pressure or enlarged ventricles (Fig. 1h). Tracer accumulation in the perivascular spaces along cortical penetrating arteries was observed in SHR but not in WKY (Fig. 2).Conclusion
Hypertensive rats showed normal-to-increased influx and decreased efflux of tracer to and from the brain parenchyma, and the enlarged perivascular spaces showed accumulation and stagnation of tracers, supporting the hypothesis of hypertension compromising the glymphatic system. Since the glymphatic pathway assists in clearance of excess metabolites and solutes like amyloid-ß and tau from the brain parenchyma, this may indicate a novel model for the role of hypertension in the pathobiology of SVD and dementia.Acknowledgements
This study was supported by NIH-R01AG048769 and NIH-RF1AG053991, and a grant from the Novo Nordisk Foundation.References
1. V. Hachinski, Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke; a journal of cerebral circulation 46, 3039-3040 (2015). 2. Wardlaw, J. M., Smith, C., & Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. The Lancet Neurology, 12(5), 483-497 (2013). 3. Iliff, J. J., Lee, H., Yu, M., Feng, T., Logan, J., Nedergaard, M., & Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. The Journal of clinical investigation, 123(3), 1299-1309 (2013). 4. Lee, H., Xie, L., Yu, M., Kang, H., Feng, T., Deane, R., ... & Benveniste, H. The effect of body posture on brain glymphatic transport. The Journal of Neuroscience, 35(31), 11034-11044 (2015). 5. Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033-2044 (2011). 6. Ritter, S., & Dinh, T. T. Progressive postnatal dilation of brain ventricles in spontaneously hypertensive rats. Brain research, 370(2), 327-332 (1986).
Figures