4130
HYPOTHALAMIC-PITUITARY-GONADAL AXIS DYSREGULATION ALTERS RESTING STATE FUNCTIONAL CONNECTIVITY IN A MOUSE MODEL OF ALZHEIMER’S DISEASE1Bioimaging Lab, Antwerp University, Antwerp, Belgium, 2Laboratory of Veterinary Physiology and Biochemistry, Antwerp University, Antwerp, Belgium, 3Mathematics, Leiden University, Netherlands, 4Paul Flechsig Institute for Brain Research, University of Leipzig, Leipzig, Germany, 5Experimental Cell Transplantation Group, Antwerp University, Antwerp, Belgium
Synopsis
Dysregulation of hypothalamic pituitary gonadal (HPG) axis signaling with menopause is considered as a risk factor for Alzheimer’s disease (AD). Menopause leads to decreased sex steroid signaling and increased luteinizing hormone signaling which may have profound effects on many cellular processes that predispose to neurodegeneration and impairment in cognitive function. The effects of amyloid production on resting state BOLD fMRI using functional connectivity analysis in a mouse model of AD have been previously published. However how HPG axis dysregulation affect resting state functional connectivity in a mouse model of AD has not been studied. Here we show that ovariectomized AD mice, a commonly used animal model to study menopause related hormonal changes in the HPG axis, exhibit alterations in resting state connectivity in the mouse default mode network connectivity. These findings establish a causal link between AD and HPG axis dysregulation.
Introduction
The hypothalamic-pituitary-gonadal axis (HPG-axis) imbalance (observed after post-menopause), which causes accelerated aging, is considered as an important risk factor for Alzheimer’s disease (AD)[1]. However, little is known about how dysregulation of the HPG axis hormones (i.e. estrogen) affects the integrity of the resting state functional connectivity, and how these changes contribute to the disease progression. Among the brain networks, the default mode network (DMN) activity is one of the most widely studied resting state functional networks due to its sensitivity to pathological alterations in the brain. In this study, we used resting state blood oxygen level dependent (BOLD) functional magnetic resonance imaging (rsfMRI) to longitudinally monitor brain functional connectivity changes in the DMN-like brain regions in an AD mouse model (i.e. Tg2576 mice), which develop amyloid deposits in the cortical and limbic regions by 9 months of age [2]. We hypothesized that the ovariectomized (OVX) mice, the most commonly used pre-clinical model to study impact of the dysregulation of the HPG axis, will show altered resting state functional connectivity due to amyloid pathology, low peripheral estrogen levels, and elevated inflammatory markers .Methods
Experimental design: Tg2576 (TG) and age-matched wild-type (WT) littermates were used in this study [2]. A group of WT (n=24) and TG (n=23) mice were either sham operated or ovariectomized (OVX, bilateral removal of ovaries) (at the age of 3.5 months) after an initial baseline scan at the age of 3 months (M) . The mice were re-scanned at the age of 6, 8, 14 and 18 M (post-operation time points). MRI: RsfMRI were acquired on a 9.4T Biospec MRI system (Bruker) using following imaging parameters: TE, 15ms; TR, 2s; FOV, 20mm; Matrix, 128x64; nr of axial slices, 16. ECG and respiration rate were monitored and body heat was kept constant by a warm air system. Histology: An extensive histological and plasma hormone analysis were performed to detect following parameters: gliosis, Aβ level and luteinizing hormone levels (data is not shown)). Data Analysis: Longitudinal rsfMRI data were analyzed using SPSS’s linear mix model with scaled identity (covariance type) and restricted maximum likelihood estimation as described earlier [3]. For hypothesis testing, significant effects and interactions were explored using least significant difference test. The mean difference is significant at the level of p=0.05.Results
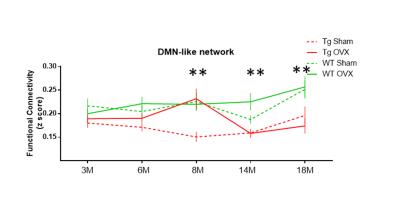
Figure 1 shows the comparison of group functional connectivity (FC) matrices, representing the correlation in BOLD time series between selected DMN-like brain regions, of WT and TG mice for different ages after post-operation (i.e. sham or OVX). The comparison of group means showed age-dependent significant differences between genotypes (i.e. TG and WT) and groups (i.e. TG-OVX, TG-sham, WT-OVX, WT-sham). In addition statistically significant time affect was found (P<0.05). The significant changes with time (i.e. age) are as follows (see Figure 2): 8 months old TG-OVX mice depicted elevated functional connectivity levels compared to 14 and 18 months old TG-OVX (P=0.019 & P=0.036 respectively), and 18 months old WT-sham group have higher functional connectivity levels compared to 6 months old WT-sham group (P= 0.035). We found following statistically significant group differences for specific ages (see Figure 2): at 8 months of age, TG-sham group showed decreased functional connectivity levels compared to WT-sham group (P= 0.002) while TG-OVX group depicted hypersynchronous FC compared to TG-sham group (P=0.003); at 14 months of age, the TG-sham group showed decreased FC levels compared to WT-sham group (P=0.045) and TG-OVX group showed decreased FC compared to WT-OVX group (P=0.005); at 18 months of age, TG-sham group showed decreased FC compared to WT-sham group (P=0.05) and TG-OVX group showed decreased FC compared to WT-OVX group (P= 0.001).Discussion and Conclusion
In our study TG-OVX mice showed hypersynchronous FC within DMN brain regions compared to TG-Sham at age 8 M. Since both TG-OVX and TG-sham groups develop amyloid pathology, we speculated that a decrease in peripheral estrogen level (due to ovariectomization) when coupled with amyloid pathology may cause an imbalance between the excitatory and inhibitory neurotransmitter signaling. TG-OVX group showed significant FC differences with age (between 8M and 18M) (Figure 2) suggesting that the impact of HPG axis dysregulation on the brain functional networks in TG-OVX group is more evident early during the disease progression when there are less amyloid deposits. To conclude, our results suggest that HPG axis dysregulation is associated with brain functional connectivity changes in TG mice which develop AD like pathology. Investigating the underlying mechanisms of hypersynchronous FC within DMN like structures in TG mice may shed light on why post-menopausal women have higher risk for AD and help for developing new treatment strategies.Acknowledgements
Acknowledgement: StichtingAlzheimer Onderzoek (SAO-FRA, 14027), IWT (13160), Scientific Research Flanders (FWO) (grant agreement G.0D76.14, G.0587.14.),postdoctoral FWO number (no 12S4815N),INMiND(278850).We acknowledge Caroline Guglielmetti and Cynthia Anckaerts for their technical help.References
[1] Blair et al., Frontiers in Endocrinology 2015; [2] Hsiao K., Chapman P., Nilsen S., et al., Science 1996. 274:99-103; [3] Duriscki D.A., Soleman S., Moon LDF. et al., Nature Protocols 2016: 11, 1112-1129.Figures