4129
A multi-modal study of the neural mechanisms behind the beneficial effects of repetitive TMS over the precuneus of MCI patients1University of Rome "Roma Tre", Rome, Italy, 2Neuroimaging Laboratory, Santa Lucia Foundation, Rome, Italy, 3Clinical Imaging Sciences Centre, University of Sussex, Brighton, United Kingdom, 4Non-Invasive Brain Stimulation Unit, Santa Lucia Foundation, Rome, Italy, 5Stroke Unit, Policlinico Tor Vergata, Rome, Italy
Synopsis
We present a double-blind randomized cross-over clinical study that aims to investigate the efficacy of two weeks of repetitive transcranial magnetic stimulation (TMS) in modulating cognitive performances in patients with mild cognitive impairment (MCI) and to characterize in vivo brain connectivity changes in MCI patients after rTMS. We used a multi-modal approach based on behavioural tests, TMS-EEG, DWI and fMRI. The behavioural and neurophysiological results support the role of medial parietal region in memory process. Moreover, our findings suggest that TMS may be a potential effective strategy in treatment of MCI patients for whom, currently, there is no available therapy.
Purpose
Mild cognitive impairment (MCI) has been identified as the earliest condition associated with an increased risk for developing Alzheimer's Disease (AD). The most common clinical presentation of MCI is associated with memory loss as the predominant symptom (amnesic MCI). Previous studies have shown that MCI is characterized by altered connectivity in the posterior part of the Default Mode Network (DMN)1. The application of transcranial magnetic stimulation (TMS) over the precuneus (PC) was shown to be associated with an improvement in episodic memory suggesting a direct implication of the PC in successful context-dependent retrieval2. The present double-blind randomized cross-over clinical study aims to investigate the efficacy of two weeks of repetitive TMS in modulating cognitive performances in patients with MCI and to characterize in vivo brain connectivity changes in MCI patients after rTMS.Methods
Fourteen MCI patients were enrolled in the study. TMS was applied over PC at 20 Hz, in a 10-session course over two weeks in a sham-controlled crossover design (fig. 1). Subjects were randomly assigned to real stimulation or sham stimulation. A 2-week washout period was applied following which subjects were crossed over to the alternate treatment for additional two weeks. Neuropsychological tests and TMS-EEG co-registrations were obtained at baseline and after two weeks of treatment for real and sham condition. Eight out of 14 patients also had MRI, including T1-weighted volumes, resting state fMRI (TR=2.08 s; TE=30 ms) and diffusion-weighted imaging (DWI, (TR=7000 ms, TE=85 ms, 61 directions, b-factor=1000 smm-2) at baseline and follow-up. In order to ensure targeting of the PC, cortical sources were estimated from the TMS-EEG data and the response to the stimulation of the PC was tested against the baseline using a t-test. To evaluate the behavioural effects of rTMS treatment, a modified version of Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite (ADCS-PACC)3 was adopted: the ADCS-PACC combines tests that assess episodic memory, timed executive function, and global cognition. The effects of the treatment were evaluated also using the TMS-evoked responses over each stimulation site with a spatio-temporal-domain analysis to assess cortical excitability at global level. T1 volumes were pre-processed using FreeSurfer and parcellated using the Desikan-Killiany atlas. Diffusion images were co-registered to mean b0 volume in order to minimize distortions and then processed using tensor fitting and deterministic tractography by means of Diffusion Toolkit. The reconstructed streamlines were linearly co-registered to the anatomical space and for each subject the number of streamlines between each couple of parcellated regions was counted and recorder in an adjacency matrix. Functional data were pre-processed using FSL and AROMA4 for a robust reduction of movement artifacts, and then co-registered to the anatomical space. For each region, the average BOLD time-series was computed and for each pair of regions the Pearson correlation between the averaged time-series was computed. Appropriate thresholding was used for both structural and functional connectivity5. We then assessed changes within the DMN and across-networks. For the former, we compared functional connectivity pre-post treatment differences for the DMN regions6, using the degree as a connectivity measure and the sign test for assessing significance. For the latter, anatomically-constrained functional connectivity7 was estimated evaluating functional connectivity in presence of structural connections estimated by DWI. Differences were assessed using network-based statistics (NBS)8 using two different thresholds.Results
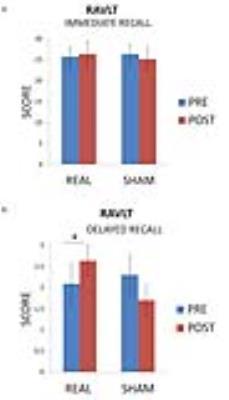
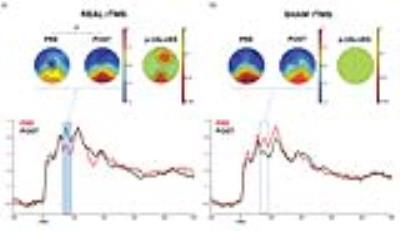
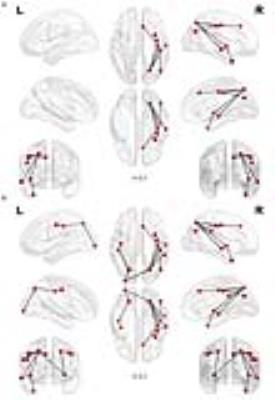
The patients demonstrated an improvement in episodic memory performance following real stimulation compared to sham stimulation (p<0.05, fig. 2). Source analysis showed a significant response of the PC compared to baseline (p<0.01) and the whole treatment produced significant increase of TMS evoked activity over a specific time window (60 ms after TMS) over a fronto-parietal cluster of electrodes (p<0.01, fig. 3). No significant difference was observed comparing pre-post degrees differences for the regions of the DMN. Anatomically-constrained connectivity has instead revealed decreased connectivity in a specific fronto-parietal network (t=3.1, p=0.0051, fig. 4a; t=2.1, p=0.0192, fig. 4b) when comparing pre-post sham and effective stimulation conditions.Discussion
The behavioural and neurophysiological results support the role of medial parietal region in memory process. Moreover, the MRI results indicate decreasing local connectivity beyond the DMN, an effect that potentially compensates the AD-related increased local connectivity9,10.Conclusion
Used together, TMS, structural and fMRI techniques, offer a unique opportunity to assess in vivo functional and structural brain connectivity in healthy as well as in diseased brains. These findings also suggest that TMS may be a potential effective strategy in treatment of MCI patients for whom, currently, there is no available therapy.Acknowledgements
This study was funded by the Italian Ministry of Health (RF10.047).
References
1. Bozzali et al. Damage to the cingulum contributes to Alzheimer's disease pathophysiology by deafferentation mechanism. Human Brain Mapping 2011, 33(6): 1295-308; 2. Bonnì et al. TMS evidence for a selective role of the precuneus in source memory retrieval. Behavioural Brain Research 2015, 282: 70-75; 3. Donohue et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014, 71(8): 961-70; 4. Pruim et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 2015, 112: 267-77; 5. Fallani et al. Graph analysis of functional brain networks: practical issues in translational neuroscience. Phil Trans R Soc B 2014, 369: 20130521; 6. Power et al. Functional network organization of the human brain. Neuron 2011, 72(4): 665-78; 7. Liégeois et al. Cerebral functional connectivity periodically (de)synchronizes with anatomical constraints. Brain Structure and Function 2016, 221(6): 2985-97; 8. Zalesky et al., Network-based statistic: identifying differences in brain networks. Neuroimage 2010, 53(4): 1197-207; 9. Jones et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain 2016, 139(2): 547-62; 10. Zhang et al. Enhanced resting-state functional connectivity between core memory-task activation peaks is associated with memory impairment in MCI. Neurobiology of Aging 2016, 45: 43-49.Figures