4125
Diagnostic Decision Support in Alzheimer’s Disease: Predicting Typical and Mixed Forms from Combined Routine Brain Volumetry and Cognitive Assessment1Department of Radiology, CHUV, Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3LTS5, EPFL, Lausanne, Switzerland, 4Leenaards Memory Centre, CHUV, Lausanne, Switzerland
Synopsis
We implemented an automated classifier using T1-weighted magnetic resonance imaging-based brain volumetry and the Montreal Cognitive Assessment test to predict whether patients of a University Memory Clinic with suspected neurocognitive disorders have subjective complaints, or suffer from either typical or mixed forms of Alzheimer's disease. The classifier achieved an accuracy of 80.8% and was found to require both psychometric and brain morphometric data to perform best.
Purpose
There is increasing evidence that T1-weighted MR-based brain morphometry can help in the differential diagnosis of various forms of neurocognitive disorders [1]. We investigated the automated classification of Alzheimer's disease (AD) versus a mixed form of dementia, in which AD and vascular dementia occur at the same time, in particular with the presence of diffuse microvascular lesions. Our aim is to evaluate the ability of a simple classification algorithm using multimodal information readily available in a standard memory clinic to predict whether patients with suspected AD have subjective complaints or suffer from either the typical or the mixed form of AD.Methods
We collected MPRAGE scans and Montreal Cognitive Assessment (MoCA) scores from 59 routinely enrolled patients in the Leenaards Memory Center (age range: 50-89 years, mean age: 74 years, 54% women). Patients gave written consent for research studies. Diagnosis was performed based on ICD10 categories. 16 patients were diagnosed with subjective complaints, 19 with typical AD, and 22 with mixed dementia.
The MorphoBox prototype [2] was used to assess, for each patient, the left and right temporal gray matter (GM) volumes as well as the overall white matter (WM) abnormality volume, in proportion of the total intra-cranial volume. WM abnormalities were detected by evaluating the difference between the posterior and prior probability maps described in [2] and applying a constant threshold value of 0.6. Gaussian naive Bayes three-class classifiers [3] were implemented using the Scikit-learn package [4]. Three distinct classifiers were evaluated: one using six predictive features considered as continuous variables (age, gender, MoCA score, left and right temporal lobe volumes, and white matter abnormality volume); one using the same features except MoCA; and, finally, one using age, gender and MoCA as features. In the sequel, these classifiers are referred to as the “6-feature”, “5-feature” and “3-feature” classifiers, respectively. Classification accuracy, defined as the percentage of correct classification, was evaluated for each classifier using the leave-10%-out cross-validation method.
Results
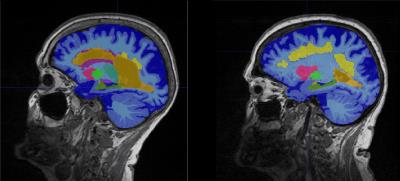
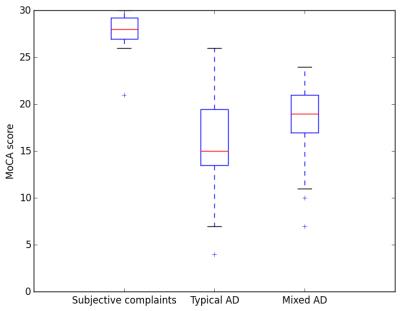
Example MR slices with MorphoBox segmentation results overlaid are shown in Figure 1 for two patients, one with typical AD and one with mixed dementia. The distributions of MoCA scores within groups are represented using box plots in Figure 2, showing that MoCA scores are lower in both typical and mixed AD patients than in patients with subjective complaints, but also considerably more dispersed. The classification accuracy was found to be 80.8% using the 6-feature classifier, 76.8% using the 5-feature classifier, and 73.9% using the 3-feature classifier, which is significantly above chance in all cases.
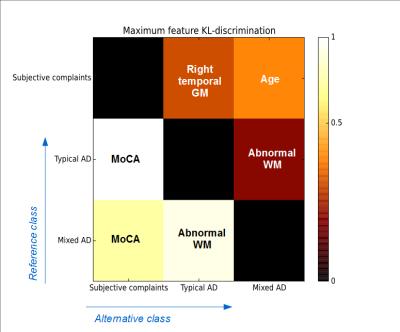
To further understand the role of each feature in the classification, we represent in Figure 3 the most discriminant features among the six selected ones in the sense of the Kullback-Leibler divergence [5], for the different pairwise class comparisons. For instance, the feature that best detects typical AD in comparison with subjective complaints turned out to be the MoCA score, and the same was observed for the detection of mixed AD in comparison with subjective complaints. However, the most discriminant feature to detect typical AD in comparison with mixed AD was the abnormal WM volume, and this was also true the other way round.
Discussion and Conclusion
The observation that the best classification performance was obtained using the 6-feature classifier indicates that both MoCA and brain volumetry play a role in the classification, and therefore bring complementary information to the differential diagnosis. This finding is confirmed by our statistical discrimination analysis, which shows that, while the MoCA score is the driving feature to discriminate between subjective complaints and AD, the abnormal WM volume is essential to discriminate between typical and mixed forms of AD.
This work suggests that the development of clinical decision support methods for the differential diagnosis of dementia benefits from multimodal information, e.g. from psychometric tests and brain morphometry. In the future, we will consider other types of dementia as well as other psychometric and brain morphometric features, for which larger patient databases will be required.
Acknowledgements
No acknowledgement found.References
[1] Goto, H., Ishii, K., Uemura, T., Miyamoto, N., Yoshikawa, T., Shimada, K., & Ohkawa, S. (2010). Differential diagnosis of dementia with Lewy Bodies and Alzheimer Disease using combined MR imaging and brain perfusion single-photon emission tomography. American Journal of Neuroradiology, 31(4), 720-725.
[2] Schmitter, D., et al (2015). An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. NeuroImage: Clinical, 7, 7-17.
[3] Murphy, K. P. (2006). Naive bayes classifiers. University of British Columbia.
[4] Pedregosa, F., et al (2011). Scikit-learn: Machine learning in Python. Journal of Machine Learning Research, 12, 2825-2830.
[5] Cover, T. M., & Thomas, J. A. (2012). Elements of information theory. John Wiley & Sons.
Figures