4119
Degeneracy between apolipoprotein E ε3 and ε4 alleles predicts elderly episodic memory variation: a longitudinal study with independent validation1Department of Neurology, Affiliated ZhongDa Hospital, Neuropsychiatric Institute and Medical School of Southeast University, Nanjing, People's Republic of China, 2Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
We reported distinct EM neural correlates among APOE alleles in elderly subjects but still unclear whether and how the APOE ε4 carriers’ distinct EM neural correlates contribute to an increased risk of AD onset. This study found that higher HFC strength in the EM neural correlates correlated with better longitudinal EM performance in the APOE ε3ε3 subjects but associated with inferior longitudinal EM performance in the ε4 carriers. These findings indicate that the degeneracy of EM function advance differential AD onset risk among APOE alleles, and provide a potential tool to predict APOE ε4 carriers with impending cognitive decline.
Introduction
We reported that, in a cognitively normal (CN) elderly cohort, different apolipoprotein E (APOE) alleles exhibited distinct neural correlates of episodic memory (EM) function in the hippocampal functional connectivity (HFC) network.1 This EM neural correlate divergence indicates the neural degeneracy of EM function among APOE alleles, which refers to a many-to-one structure-function relationship in EM function.2 However, whether and how the APOE ε4 carriers’ distinct EM neural correlates contribute to an increased risk of Alzheimer’s disease (AD) onset remains unknown. To address this question, here, we followed up with this cohort and additionally included amnestic mild cognitive impairment (aMCI) subjects.Methods
Subjects. In this study, we included 88 CN subjects (43 APOE ε3ε3 subjects and 45 APOE ε4 carriers) and 64 aMCI subjects (41 APOE ε3ε3 subjects and 23 APOE ε4 carriers). We assessed EM function and performed resting-state functional MRI scans (Siemens Verio 3.0T MRI scanner) on all subjects. Among CN subjects, we followed up with 27 APOE ε3ε3 subjects and 21 APOE ε4 carriers at an average interval of three years. These subjects underwent EM tests at the follow-up visits.
Data processing. A seed-based approach established bilateral hippocampal functional connectivity (HFC) networks. A within-group, voxelwise multivariate linear regression analysis between the HFC strength and EM score detected the EM neural correlates in CN-APOE ε3ε3 and CN-APOE ε4+ groups.
Statistical analysis. We performed statistical analyses within APOE ε3ε3 subjects and APOE ε4 carriers, respectively. We compared HFC strengths between aMCI and CN subjects, and extracted the overlapping regions between decreased HFC in aMCI and EM neural correlates in CN. Then, we used two linear regression models to investigate the association between baseline HFC in the overlapping regions and longitudinal EM performance. First, a multivariate regression analysis between baseline HFC and the EM score at the follow-up visit was performed, controlling for effects of the baseline EM score, follow-up interval, age, gender, education years, and family history. Second, a simple regression analysis detected the correlation between baseline HFC and the annual change in EM score. Finally, these regression analyses were performed on subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset (24 APOE ε3ε3 subjects and 14 APOE ε4 carriers) to independently validate our results.
Results
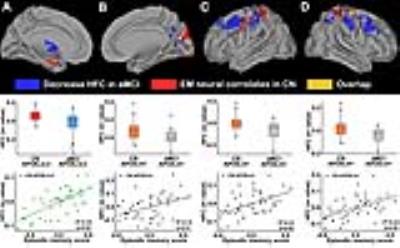
Baseline results. We observed that the EM neural correlates patterns in CN subjects were spatially similar, with decreased HFC patterns in aMCI subjects within each APOE allele group (Figs. 1 and 2). For example, within the APOE ε3ε3 subjects, CN subjects exhibited positive EM neural correlates in the Papez circuit regions (bilateral thalamus and medial temporal lobe, Fig.1A), and aMCI subjects showed decreased HFC in the bilateral thalamus and lateral temporal lobe (Fig. 1B); the two patterns overlapped in the thalamus (Fig. 2A). Within the APOE ε4 carriers, CN subjects exhibited positive EM neural correlates in the bilateral cuneus and sensorimotor regions (Figs. 1C and 1E), and aMCI subjects showed decreased HFC primarily in these regions (Figs. 1D and 1F); the two patterns overlapped in the right cuneus (Fig. 2B) and bilateral sensorimotor regions (Figs. 2C and 2D).
Longitudinal results. The APOE ε3ε3 subjects showed a positive correlation between baseline HFC strength in the thalamus overlap regions and the follow-up EM score (Fig. 3A), whereas the ε4 carriers exhibited a negative correlation between baseline HFC strength in the cuneus and sensorimotor regions and the follow-up EM score (Fig. 3B). Furthermore, baseline HFC negatively correlated with the annual change in EM score in the APOE ε4 carriers (Fig. 3C). The higher the HFC value at baseline, the greater the rate of EM score decrease at follow-up. More importantly, such associations were independently validated in the ADNI datasets, wherein baseline HFC strength in the EM neural correlates negatively correlated with both the follow-up EM score (Fig. 3D) and the annual change in EM score (Fig. 3E). The higher the HFC strength at baseline, the more inferior the EM performance at follow-up.
Discussion and conclusion
The major finding of this study is that distinct EM neural correlates between APOE ε3ε3 subjects and APOE ε4 carriers have opposite contributions to their longitudinal EM performances. More importantly, the detrimental effect of high HFC strength in the APOE ε4 carriers was independently validated by the ADNI dataset. These findings indicate that the degeneracy of EM function advance differential AD onset risk among APOE alleles. Moreover, it provides a potential predicting tool to select APOE ε4 carriers with impending cognitive decline, thus facilitating secondary prevention of AD.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81500919, 81420108012, and 91432000), the USA National Institutes of Health (R44AG035405, Brainsymphonics, LLC; UF1AG051216), and the Key Program for Clinical Medicine and Science and Technology, Jiangsu Province, China (BL2013025 and BL2014077). We sincerely thank Ms. Lydia Washechek, B.A., for editorial assistance.
References
1. Shu H, Chen G, Wang Z, et al. Divergent episodic memory networks among apolipoprotein E alleles in cognitively normal elderly. ISMRM 23rd Annual Meeting. Program number: 2218.
2. Edelman GM, Gally JA, 2001. Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences of the United States of America 98, 13763-13768.
Figures