4117
Early Prediction of Cognitive Deficits in Very Preterm Infants using Machine Learning Algorithms1Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States
Synopsis
By school age, 30-50% of very preterm infants exhibit cognitive deficits. Unfortunately, cognitive deficits cannot be reliably diagnosed until 3 to 5 years of age. These early years are now recognized as critical for neuroplasticity when early intervention therapies can enhance infants’ ability to reach their full cognitive potential. Diffuse white matter abnormality (DWMA) is seen on term-equivalent age MRI in 50-75% of very preterm infants and is predictive of cognitive deficits. In this study, we examined features of DWMA and conducted personalized prediction in very preterm infants, soon after birth, using machine learning algorithms.
PURPOSE
Approximately half a million of the 4 million total births are premature each year in the United States. The subgroup of very preterm births (at ≤32 weeks gestational age) comprise approximately more than 25% of all preterm births1. Very preterm infants are at high risk for neurodevelopmental impairments. By school age, 30-50% of them exhibit cognitive deficits2. Unfortunately, cognitive deficits cannot be reliably diagnosed until 3 to 5 years of age. These first few years are now recognized as critical for neuroplasticity when early intervention therapies can enhance infants’ ability to reach their full cognitive potential. Diffuse white matter abnormality (DWMA) is seen on term-equivalent age MRI in 50-75% of very preterm infants and is predictive of cognitive deficits. Histologically, it exhibits pathologic characteristics that are both overlapping and distinct from infants with periventricular leukomalacia3. We previously have demonstrated that DWMA, detected automatically on conventional T2-weighted MRI and diffusional parameter maps at around term-equivalent age4, 5, is a robust predictor of later cognitive deficits using linear regression analysis. In this study, we employed machine learning algorithms, especially support vector machine (SVM), to further improve prediction accuracy and facilitate personalized prediction soon after birth, in very preterm infants. SVM shows advantage in investigating complex patterns in high-dimensional neuroimaging data6. This technique is well-suited to accommodate and analyze multi-dimensional neuroimaging data, identifying data-driven decision boundaries which can be used to maximally separate populations of interest.METHODS
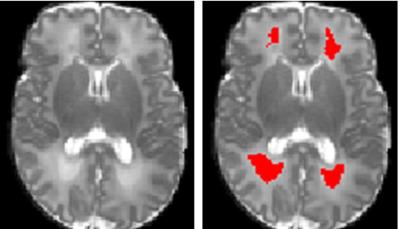
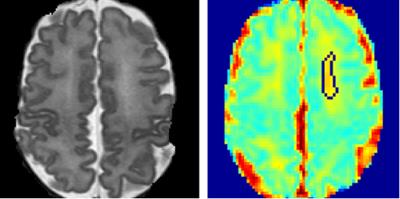
The study population was derived from an imaging cohort of 36 extremely low birth weight (ELBW; birth weight ≤1000 g) infants from the Children’s Memorial Hermann Hospital. All infants were imaged with a 3 T Philips scanner using a dual-echo fast-spin-echo sequence with use of an eight-channel SENSE-compatible phased array receive head coil. The imaging parameters used were: TE1 = 8.75ms; TE2 = 175 ms; TR = 10,000 ms; flip angle = 90°; FOV = 180×180 mm2; imaging matrix = 256×256 mm2; slice thickness = 2 mm. Single-shot DTI was acquired in 15 noncollinear directions using the parameters: TR = 6000 ms; TE = 61 ms; FOV = 180 × 180 mm2; imaging matrix = 128 × 128 mm2; slice thickness = 2 mm; high b-value = 800 s/mm2; low b-value = 0; factor = 2. All discharged ELBW infants were invited back at 18-22 months corrected age and administered a standardized Bayley Scales of Infant and Toddler Development III (BSID-III). The BSID-III cognitive and language subtests (on a scale of 50 to 150, with a mean of 100 and 150 indicating the most advanced development) were administered. We defined low BSID-III scores as mean cognitive and language scores less than 80. The DWMA are detected on T2-weighted images (Fig.1). The detected binary DWMA masks were then superimposed on the parameter maps (Fig.2) to calculate the regional average, variance, and sum/total values of mean diffusivity, axial diffusivity, and radial diffusivity measures in detected DWMA regions5. Given the small data set and imbalance, we adopted the adaptive synthetic minority over-sampling technique7 to augment training set, resulting in 60 subjects in total (30 in each category). Principle component analysis (PCA) was implemented to select features. Dimension of features were reduced from 10 to 3, which add up to more than 95% of the full variance of the data and hence dominate the explained variance ratio of the PCA. We implemented SVM with linear kernel function for the classification.RESULTS
Reported SVM results include classifier accuracy, sensitivity, specificity and p-value for binomial probabilities. Sensitivity is the ability of the classifier to correctly identify those high risk infants, whereas specificity is the ability of the classifier to correctly identify those low risk infants. The p-values represent the probability of observing the reported accuracy (number of correct classification trials) by chance based upon the binomial distribution for the given sample size. The classification of individual infant at high risk of cognitive deficits was tested with a 100-times leave-one-out cross validation and emerged an accuracy of 85%, sensitivity of 100% and specificity of 71%, p < 0.00001.CONCLUSIONS
This study shows that structural- and DTI-based objectively quantified DWMA is an early and significant predictor of cognitive deficits at 18 to 22 months corrected age. Binary SVM was applied to reliably classify very preterm infants into high-risk vs. low-risk of cognitive deficits at term-equivalent age. These findings lend further evidence that DWMA is associated with abnormal neurodevelopment at 2 years of age in very preterm infants. A larger study with school-age neurocognitive follow-up is important to validate our findings.Acknowledgements
This work was supported by the NIH-NINDS, R01 NS094200-01. The funding agency played no role in the design, conduct, or analysis of the trial. The authors take full responsibility for the integrity of the data and analyses.References
1. Glass HC, Stayer SA, Brett C, et al., Outcomes for extremely premature infants. Anesth Analg, 2015; 120(6): 1337–1351.
2. Potharst ES, van Wassenaer AG, Houtzager BA, et al., High incidence of multi-domain disabilities in very preterm children at five years of age. J Pediatr, 2011; 159: 79-85.
3. Parikh NA, Pierson CR, Rusin JA. Neuropathology associated with diffuse excessive high signal intensity abnormalities on magnetic resonance imaging in very preterm Infants. Pediatr Neurol. 2016 Jul 21 [Epub ahead of print]
4. He L and Parikh NA. Atlas-guided quantification of white matter signal abnormalities on term-equivalent age MRI in very preterm infants: findings predict language and cognitive development at two years of age. PLoS ONE. 2013; 8(12):e85475.
5. Parikh NA, He L, Bonfante-Mejia E, et al., Automatically quantified diffuse excessive high signal intensity on MRI predicts cognitive development in preterm infants. Pediatr Neurol. 2013; 49(6):424-30.
6. Pereira, F., Mitchell, T., Botvinick, M., Machine learning classifiers and fMRI: a tutorial overview. 2009; NeuroImage 45, S199–S209.
7. He H and Garcia EA, Learning from imbalanced data, IEEE Transactions on Knowledge and Data Engineering. 2009; 21(9) 1263-1284.