4110
Deep grey matter brain alkalosis in neonatal encephalopathy measured using 31P ISIS is associated with seizure burden and poor outcomes1Medical Physics and Biomedical Engineering, UCLH NHS Trust, London, United Kingdom, 2Neonatology, UCLH NHS Trust, London, United Kingdom, 3Institute of Neurology, University College London, 4Institute for Women's Health, University College London
Synopsis
31P image selected in vivo spectroscopy was used to measure deep grey matter intracellular pH in 43 neonates with hypoxic-ischaemic encephalopathy. All neonates had previously received therapeutic hypothermia, the current standard of care. Brain alkalosis was associated with poor outcomes reproducing what was found from whole-brain pH measurements in the pre-cooling era. Brain alkalosis was associated with increased seizure burden, the first time this has been shown in babies. Avoiding brain alkalosis could be a new objective for treating seizures and for neuroprotection in neonatal encephalopathy.
Purpose
Hypoxic-ischaemic neonatal encephalopathy (NE) is responsible for one million annual deaths world-wide while a similar number of survivors develop life-long neuro-disabilities. Brain alkalosis determined by whole brain 31P has previously been shown to be related to poor outcome in NE in uncooled neonates1. However therapeutic hypothermia has since become the standard of care in the developed world. Brain alkalosis has also been associated with increased seizure burden in a rodent model of NE2. Using 31P image selected in vivo spectroscopy (ISIS) the aims of the study were: (i) to analyse deep grey matter (DGM) intracellular pH (pHi) as a reliable biomarker of brain injury in NE following therapeutic hypothermia, and (ii) to assess the association between alkalosis and seizure burden. The hypothesis being that subjects with zero or low seizure burden would have a lower pHi than those with a longer seizure burden. The precision of the pHi determination was evaluated by simulating and fitting spectra with different signal-to-noise ratios (SNRs).Methods
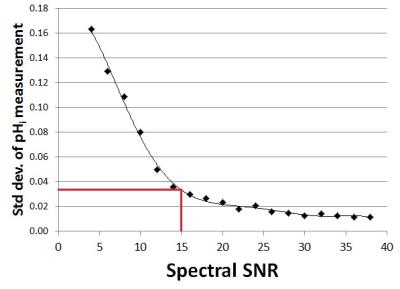
The study group consisted of 43 newborn infants with NE who received therapeutic hypothermia. Ethical approval and informed consent were obtained. Mean (± SD) gestational age was 39.8 ± 1.7 weeks and mean (± SD) birth weight 3380 ± 613 g. Subjects were MR scanned between 2-15 days of life using a 3T scanner (Philips Achieva, Philips Medical Systems, Best, The Netherlands). 31P ISIS was performed with a neonatal volume coil (Rapid Biomedical, Rimpar, Germany) with a DGM voxel covering the basal ganglia (40 x 25 x 25 mm), (TR= 2300 ms, NSA = 45 measurements x 8 ISIS phase cycles, adiabatic pulses, spectral width 3000 Hz, 2048 points, scan time 15 mins:24 secs, centre frequency between γ-ATP and α-ATP. Spectra were fitted JAVA‐based magnetic resonance user interface (jMRUI) v.4 software and quantified using a nonlinear least‐squares algorithm AMARES3. pHi was determined using the chemical shift between inorganic phosphate (Pi) and phosphor creatine (PCr) (Figure 1) using a standard calibrated version of the Henderson–Hasselbalch equation (inset Figure 1)4. 1H MRS data (PRESS, TE=288 ms, TR=2200 ms, NSA=128) were also acquired in a thalamic voxel (15 x 15 x15 mm) to yield the validated outcome biomarker Lactate/N-acetyl-aspartate (Lac/NAA) peak-area ratio5. Both voxel positions are shown in Figure 2. Seizure burden was measured from electroencephalographic recordings on ward over the first 90 hours of life6. The effect of spectral SNR on the precision of the pHi determination was evaluated using simulated spectra . Firstly the SNRs of the 43 acquired spectra were measured. SNR was defined as PCr peak height (set to 0 ppm) divided by the SD of the outer parts of the spectrum away from the peaks (10-20 ppm and -20 to -30 ppm). A representative noise-free base spectrum was obtained from an AMARES estimate. Using Matlab Gaussian noise was added to the base spectrum in discrete steps to vary spectral SNR from 4 to 38. Fifty simulated spectra were generated at each SNR step. The 50 spectra were fitted using AMARES and the standard deviation of pHi determined as a measure of precision.Results
Brain alkalosis was associated with higher Lac/Naa peak area ratio (p=0.001) (Figure 3). All infants with a Lac/NAA>0.3 (indicating an unfavourable outcome7) had a DGM pHi >7.15 (7.16-7.47) .15/43 subjects presented with seizures (0 - 539 min). Brain alkalosis was associated with a longer seizure burden (p=0.03) (Figure 4). Figure 5 shows the standard deviation of pHi against spectrum SNR. The mean (±SD) SNR of the measured spectra was 14.7 ± 2.5 indicating a precision of better than ±0.04 pHi units.Discussion
DGM brain pHi is capable of predicting outcome, reproducing what was described by whole-brain pHi in the pre-cooling era. For the first time in babies it is shown that brain alkalosis within 15 days of life in NE is associated with seizure burden in the first 90 hours. Avoiding rebound alkalosis could be a new target for treating neonatal seizures in NE and a new avenue for neuroprotection.
Acknowledgements
We acknowledge the support of the staff of the Neonatology Unit, UCLH NHS Trust.References
1. Robertson NJ, Cowan FM, Cox IJ, et al. Brain alkaline intracellular pH and adverse neurodevelopmental outcome after neonatal encephalopathy. Ann Neurol 2002;52:732–42.
2. Helmy MM, Ruusuvuori E, Watkins PV, et al. Acid extrusion via blood-brain barrier causes brain alkalosis and seizures after neonatal asphyxia. Brain. 2012 Nov;135(Pt 11):3311-9.
3. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997; 129(1): 35–43.
4. Petroff OAC, Prichard JW. Cerebral pH by NMR. Lancet 1983;ii:105–106.
5. Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, Murad S, Omar RZ, Robertson NJ et al. 2010, Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics, 2010(125): e382-e395.
6. Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch. Dis. Child Fetal Neonatal Ed. 1995;72:F34–F38.
7. Badii S, Uria-Avellanal C, Bainbridge A, et al. PC.30 3T proton magnetic resonance spectroscopy in neonatal encephalopathy: lactate/n-acetylaspartate prognosis. Arch. Dis. Child. Fetal Neonatal Ed. 2014;99:A46.
Figures

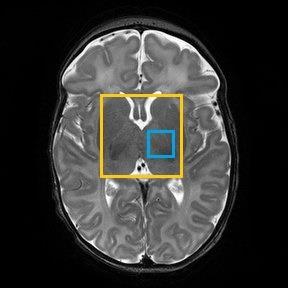
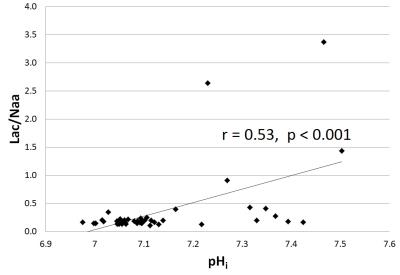
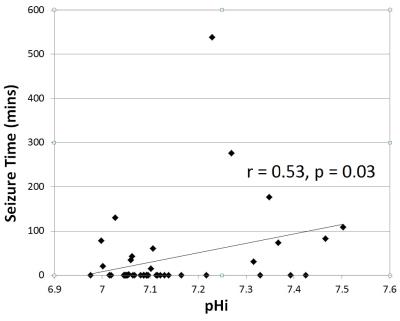
Figure 4: Seizure burden (minutes) measured over first 90 hours verses DGM pHi