4109
Amide proton transfer imaging of neonatal brain injury: a preliminary study1Shengjing Hospital of China Medical University, shenyang, People's Republic of China, 2Shengjing Hospital of China Medical University, People's Republic of China, 3Philips Healthcare, People's Republic of China
Synopsis
The environment of the brain dynamically changes with neonatal brain development, and the topic of whether the application of magnetic resonance imaging (MRI) can reflect these brain environment changes is a recent one. In recent years, a new magnetic resonance contrast technology called amide proton transfer (APT) imaging has emerged which can detect protein and peptides through the signal from water (1), reflecting in vivo pH and protein concentration at the cellular and molecular level. The so-called amide proton mainly refers to the amide proton from the free protein and the polypeptide backbones.
Abstract
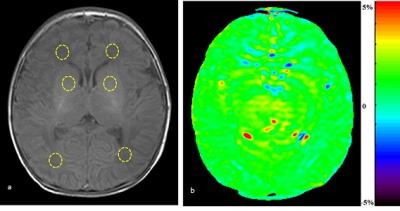
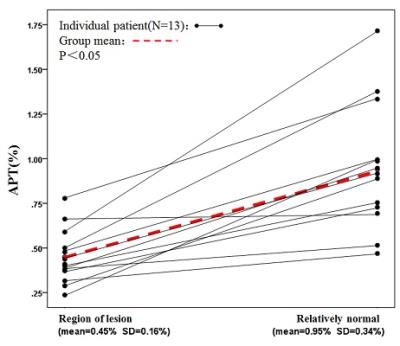
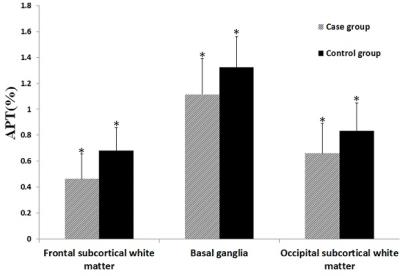
PURPOSE: To evaluate neonatal brain injury at the internal environmental level with the application of amide proton transfer (APT) imaging by measuring the APT values of the brain. METHODS: A total of 51 neonatal patients with gestational ages from 27 to 41 weeks who underwent MR examination were enrolled in the study. Using conventional MR, 38 newborns were found to have no abnormalities in the nervous system (the control group) and there were 13 newborns with different degrees of brain injury (the case group). The diagnoses were jointly made by two or more experienced radiologists. After obtaining informed consent and the clinicians’ permissions to assess the neonatal status, APT imaging was performed immediately followed by conventional MR. Thirty minutes before the examination, all newborns were given 5% chloral hydrate (50 mg/kg) via the intestinal tract, and provided with warmth and comfort. APT acquisition: All newborns were positioned at the basal ganglia level with axial T1WI and the case group increase the lesions location. In this study, an off-resonance continuous-wave RF saturation pulse with a duration of 500 ms was used for APT imaging. This study used a multi-offset and multi-acquisition APT imaging protocol, in which the APT scan and z-spectrum scan were combined. In this process, the protocol (31 offsets = 0, ±0.25, ±0.5, ±0.75, ±1, ±1.5, ±2, ±2.5, ±3 (2), ±3.25(4), ±3.5 (8), ±3.75 (4), ±4 (2), ±4.5, ±5, ±6 ppm; the numbers in parentheses were acquisitions (1 if not specified); an unsaturated image was acquired for the signal normalization) which can provide B0 inhomogeneity corrected APT images with sufficient signal-to-noise ratios within a clinically relevant time frame, which was 4 mi n 16 sec. The APT parameters were: TR=4000 ms; TE=8.1 ms; matrix=108×71, FOV=170×145 mm; thickness=5 mm. All data were processed using programs written in interactive data language (IDL; Research Systems, Inc., Boulder, CO, USA) to analyze and reconstruct a pseudo color. First, the raw image data were organized into the Z-spectrum voxel by voxel (the normalized signal intensities, Ssat/S0as a function of 31 offsets, where Ssat and S0 are the signal intensities with and without radiofrequency irradiation). Regions of interest (ROIs) were carefully chosen by two experienced radiologists, selecting bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter (Figure. 1). The case group had increased lesions area for ROIs, with the contralateral area as control. In the control group, the ROIs diameters were about 1.5 cm, whereas in the case group the ROIs should be selected within the range of disease, and no more than the edge of the lesions. Keep away from the skull and cerebrospinal fluid and the ventricles. First, in the control group, analysis was conducted of APT values in each of the parts of the bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter, to determine whether there were any differences. If there were no differences between the two sides, bilateral APT values were assigned to each group by parts respectively. The APT values of lesions and the contralateral area were compared with each other, and significant differences between lesions and contralateral relatively normal area were analyzed. Meanwhile, the APT values of bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter of the case group and control group with the same gestational age (gestational age difference ± 1 day) were applied, to analyze the statistical differences. RESULTS: In the control group, bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter had no significant difference in APT value (P>0.05). Between the different parts of the brain, APT values of preterm were lower than full-term infants (P = 0.01, P = 0.028, P = 0.003), Figure. 2. In the case group, there were significant differences in APT values between the lesion side and contralateral area, being significantly lower in lesion side than the contralateral side (P=0.000), Figure. 3. In the case group, the APT values of different parts of the brain were lower than the control group with the same gestational age (P = 0.000, P = 0.008, P = 0.01). DISCUSSION: In conclusion, utilizing endogenous protein and pH (2, 3), APT noninvasively evaluates neonatal brain injury, helping understand the mechanisms of brain injury. Furthermore, APT can be applied across the wide age-range of brain development, and this preliminary study also confirms this is achievable. CONCLUSION: From changes in the pH level in the neonatal brain, APT imaging can help understand neonatal brain injury.synopsis
The environment of the brain dynamically changes with neonatal brain development, and the topic of whether the application of magnetic resonance imaging (MRI) can reflect these brain environment changes is a recent one. In recent years, a new magnetic resonance contrast technology called amide proton transfer (APT) imaging has emerged which can detect protein and peptides through the signal from water (1), reflecting in vivo pH and protein concentration at the cellular and molecular level. The so-called amide proton mainly refers to the amide proton from the free protein and the polypeptide backbones.Acknowledgements
This study was supported by National Natural Science Foundation of China (NO. 30570541, 30770632, 81271631). Acknowledge the NIH grant P41 EB015909.References
1. Sun PZ, Benner T, Copen WA, Sorensen AG. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010; 41(10 suppl): S147–151.
2. Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wüthrich K. Recommendations for the presentation of NMR structures of proteins and nucleic acids--IUPAC-IUBMB-IUPAB Inter-Union Task Group on the standardization of data bases of protein and nucleic acid structures determined by NMR spectroscopy. Eur J Biochem. 1998; 256(1): 1-15.
3. van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med. 2011; 65(4): 927-948.
Figures