4108
Characterizing brain microstructural changes in childhood arterial ischemic stroke using multi-shell diffusion magnetic resonance imaging1Neurosurgery, Royal Children's Hospital, Melbourne, Australia, 2Neuroscience Research, Murdoch Childrens Research Institution, Melbourne, Australia, 3Developmental Imaging, Murdoch Childrens Research Institution, Melbourne, Australia, 4Stroke and aging research group, Department of Medicine, Monash University, Melbourne, Australia, 5Neurology, Royal Children's Hospital, Melbourne, Australia, 6Paediatrics, University of Melbourne, Melbourne, Australia
Synopsis
Imaging markers that can infer microstructural changes occurring in childhood arterial ischemic stroke (CAIS) may lead to a better understanding of the disease’s neurobiological substrates. We applied both the Neurite Orientation Dispersion and Density Imaging (NODDI) and the Spherical Mean Technique (SMT) diffusion compartment models to two-shell diffusion data acquired in four acute CAIS patients. We demonstrate that parameter estimates derived from the stroke-affected regions were consistent with known microstructural changes described in acute stroke histopathology. The magnitudes of parameter changes were associated with stroke severity, and the motor outcomes at follow-up.
Purpose
A strategy towards better understanding of the neurobiological substrates in childhood arterial ischemic stroke (CAIS) is to identify surrogate-imaging markers that can infer tissue microstructural changes occurring in acute stroke. Compartment-based diffusion models, such as the Neurite Orientation Dispersion and Density Imaging (NODDI)1 and the multi-compartment microscopic diffusion imaging of Spherical Mean Technique (SMT)2 enable estimations of intrinsic microstructural tissue properties on a macroscopic scale. In this study, we retrospectively applied both the NODDI and SMT modelling techniques to two-shell diffusion data acquired in four acute CAIS patients. We aim to demonstrate the model parameter estimates in the stroke-affected basal ganglia (BG) and the pyramidal tract (PT) are consistent with known microstructural changes described in acute stroke histopathology. We present preliminary evidence associating the changes in model parameters with stroke severity and the motor outcomes at follow-up.Methods
Participants
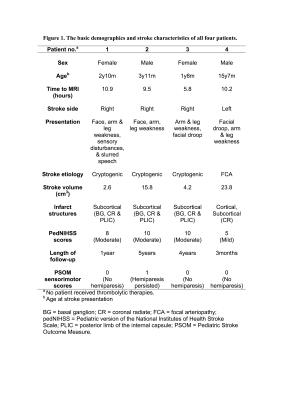
Four children with acute unilateral MCA territory ischemic stroke were retrospectively included [two males, age range 2 -16 years]. Stroke severity at presentation was assessed by the Pediatric version of the National Institutes of Health Stroke Scale (pedNIHSS) scores. Motor outcome was assessed by the Pediatric Stroke Outcome Measure (PSOM) sensorimotor subscale scores.
MRI acquisition
All data were acquired using a 32-channel head coil 3T Siemens Trio MRI scanner. The diffusion shells were: 1.) b = 3000 s/mm2, 67 non-collinear gradient directions, seven b0 volumes, TR/TE 7600/110ms; FOV = 100 x 100 mm, voxel size = 2.3 mm3 2.) b = 1000 s/mm2, 21 non-collinear gradient directions, one b0 volume; in two cases: TR/TE 9300/97ms, FOV = 100 x 100 mm, voxel size = 3 mm3; in the other two: TR/TE 5700/102ms, FOV = 100 x 100 mm, voxel size = 5 mm3.
Image processing
The diffusion data were motion and eddy current corrected, linearly co-registered and merged. To account for the different TR and TE between the two shells, each shell was normalized by the b0 image for that shell. The infarcted BG was segmented semi-automatically using the in-house software based on watershed transform. The segmentation was reflected across image midline to represent the contra-hemispheric homologs. Probabilistic PT tractography were reconstructed for both hemispheres using the high b-value shell data, and the constrained spherical deconvolution model within MRtrix.3,4 The infarct and PT masks (and their contra-hemispheric homologs) were combined to create masks representing portions of PT within and outside the infarct for parameter estimations.
The NODDI and SMT models were fitted using the Matlab (http://mig.cs.ucl.ac.uk/mig/mig/index.php/?n=Tutorial.NODDImatlab/) and C code (https://github.com/ekaden/smt). For comparison, diffusion tensor imaging (DTI) parameters were also estimated from the low b-value shell data.
Data analysis
Parameter estimates were derived from the acute infarct-affected BG, the reconstructed PT and the contra-hemispheric homologs. Contrast-to-noise ratios (CNRs) were calculated for each pair of homologous brain regions. CNRs were visualized via plotting in order to develop an understanding of possible associations between measures, outcomes and severity.
Results
Relevant clinical features are summarized in Figure 1. Patient one to three had moderate stroke and patient four had mild stroke at presentation. Hemiparesis resolved in patients one, three and four at follow-up. Patient two demonstrated persistent moderate hemiparesis at five years follow-up.
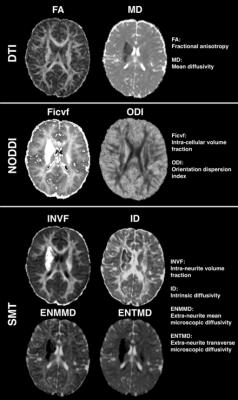
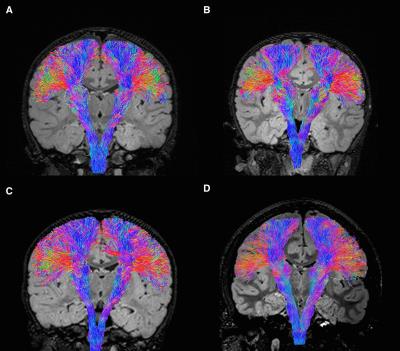
Figure 2 summarizes the parameter estimate findings. In the infarct-affected BG and the PTs from all children, both the NODDI and SMT demonstrated increased intra-cellular volume (increased Ficvf and INVF). SMT showed predominant reduced extra-cellular microscopic diffusivity (greater reduced ENMMD and ENTMD compared to ID). NODDI demonstrated increased ODI of the infarct-affected PTs, supported by the tractography appearances (Figure 3).
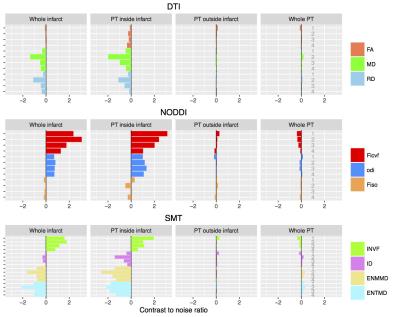
Figure 4 summarizes the CNRs from the parameter estimates in all children. Both the NODDI and SMT parameter estimates provided much greater CNRs (range 0.09 – 3.12) than the DTI model (range 0.03 – 1.99). The CNR changes for the NODDI Ficvf and ODI and all SMT parameter estimates were consistently greater for patients one to three, than patient four. Patient two had the greatest CNR changes for all SMT diffusivity estimates (ENMMD, ENTMD, and ID), comparing to the other three children.
Discussion and Conclusioin
The NODDI and SMT parameters provide complementary estimates of microstructural tissue properties in acute CAIS. Interpretation of these findings are plausibly consistent with classic acute stroke histopathological features, including cytotoxic edema, narrowed extracellular spaces, and peri-axonal / axonal swellings.5-9 Both models produce much robust infarct contrasts than DTI, which may be useful clinically in defining infarct territories. The prognostic values of the observed associations between the modeling parameter estimates with stroke severity and motor outcomes requires further elucidations in larger patient cohorts.
Acknowledgements
We thank all MRI technologists involved in MRI data acquisition, and Dr. Christopher L. Adamson, PhD, for technical support. This research was conducted within the Neuroscience Research and the Developmental Imaging research groups, Murdoch Childrens Research Institute and the Children’s MRI Centre, Royal Children's Hospital, Melbourne, Victoria. It was supported by the Murdoch Childrens Research Institute, the Royal Children’s Hospital, Department of Paediatrics The University of Melbourne and the Victorian Government's Operational Infrastructure Support Program. The project was generously supported by RCH1000, a unique arm of The Royal Children’s Hospital Foundation devoted to raising funds for research at The Royal Children’s Hospital.References
1. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61(4):1000-1016.
2. Kaden E, Kelm ND, Carson RP, Does MD, Alexander DC. Multi-compartment microscopic diffusion imaging. Neuroimage 2016.
3. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007;35(4):1459-1472.
4. Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 2004;23(3):1176-1185.
5. Benveniste H, Hedlund LW, Johnson GA. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 1992;23(5):746-754.
6. Doczi T, Schwarcz A. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke 2003;34(5):e17-18; author reply e17-18.
7. Hoehn-Berlage M, Eis M, Back T, Kohno K, Yamashita K. Changes of relaxation times (T1, T2) and apparent diffusion coefficient after permanent middle cerebral artery occlusion in the rat: temporal evolution, regional extent, and comparison with histology. Magn Reson Med 1995;34(6):824-834.
8. Kuroiwa T, Nagaoka T, Ueki M, Yamada I, Miyasaka N, Akimoto H. Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke 1998;29(4):859-865.
9. Margaritescu O, Mogoanta L, Pirici I, Pirici D, Cernea D, Margaritescu C. Histopathological changes in acute ischemic stroke. Rom J Morphol Embryol 2009;50(3):327-339.
Figures