4105
Investigating early brain-behaviour relationships in infants born preterm using whole brain, multimodal magnetic resonance imaging1Murdoch Childrens Research Institute, Melbourne, Australia, 2Department of Physiotherapy, The University of Melbourne, Melbourne, Australia, 3Newborn research, Royal Women’s Hospital, Melbourne, Australia, 4Department of Obstetrics and Gynaecology, The University of Melbourne, Melbourne, Australia, 5Department of Medicine, Monash Medical Centre, Monash University, Melbourne, Australia, 6Pediatric, Infant, Perinatal Emergency Retrieval (PIPER), Royal Children’s Hospital, Melbourne, Australia, 7Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 8Florey Institute of Neuroscience and Mental Health, Melbourne, Australia
Synopsis
The neonatal period is critical for brain development, however relationships between the brain and behaviour early in life are poorly understood. This study investigated relationships between whole brain, multimodal, quantitative magnetic resonance imaging (MRI) measures and neurobehavioural function in 257 preterm infants at term-equivalent age. Voxel-based morphometry and tract-based spatial statistics identified regions where grey and white matter volume and white matter microstructure were associated with various aspects of neurobehavioural function, with regions varying depending on the function. Thus, this study improves knowledge of brain-behaviour relationships early in life, which may help with predicting long-term outcomes and assessing early interventions to improve outcomes.
Introduction
The neonatal period is critical for brain development, which may be delayed or disrupted by preterm birth.1 By term-equivalent age (TEA), infants who were born preterm [<37 weeks’ gestational age (GA)] have poorer neurobehavioural function compared with infants born full-term (less than or equal to 37 weeks’ GA),2 which is predictive of longer-term neurodevelopmental outcomes.3 However, changes in brain structure underlying neurobehaviour at TEA are poorly understood, as no studies have used quantitative, multimodal magnetic resonance imaging (MRI) to study brain-behaviour relationships at such an early point in life. The aim of the current study was to investigate the relationship between brain structure, measured using whole brain, multimodal, quantitative MRI methods, and neurobehaviour at TEA in preterm infants.Methods
150 very preterm (VPT, <30 weeks’ GA) and 201 moderate-late preterm (MLPT, 32-36 weeks’ GA) infants were recruited from the Royal Women’s Hospital, Melbourne, into prospective cohort studies. Of those recruited, 104 VPT and 198 MLPT infants underwent MRI between 38-44 weeks’ GA inclusive. The infants also underwent neurobehavioural assessments [Prechtl’s General Movements4, Neonatal Intensive Care Unit (NICU) Network Neurobehavioral Scale (NNNS)5 and Hammersmith Neonatal Neurological Examination (HNNE)6] between 38-44 weeks’ GA. The current study included 249 infants (88 VPT and 161 MLPT) who had both structural images and neurobehavioural data, and 257 infants (88 VPT and 169 MLPT) who had both diffusion images and neurobehavioural data. Structural brain images were segmented into tissue types using the Morphologically Adaptive Neonatal Tissue Segmentation (MANTiS) technique.7 Cortical grey matter and white matter maps in neonatal template space8 were analysed using Voxel-Based Morphometry (VBM). Diffusion images were corrected for echo planar imaging and motion/eddy current distortions, and the diffusion tensor model was fitted. Diffusion tensor images were analysed using Tract-Based Spatial Statistics,9 registering all images to the most representative image in the cohort, rather than an adult template. Whole-brain, voxel-wise statistical analysis of volumes and diffusion measures was performed using non-parametric permutation based methods.10 Associations between neonatal volume and diffusion measures and neurobehavioural outcomes were analysed, adjusted for the potential confounder of age at MRI. Volume analyses were also performed with and without adjusting for intracranial volume (ICV). Additionally, differences in brain-behaviour relationships between VPT and MLPT infants were investigated by interaction analyses. Results are reported at p<0.05 following 5000 permutations, threshold-free cluster enhancement and family-wise error rate correction. Regions of statistical significance were localised to anatomical brain regions and tracts by visual inspection and comparison against a neonatal atlas.11Results
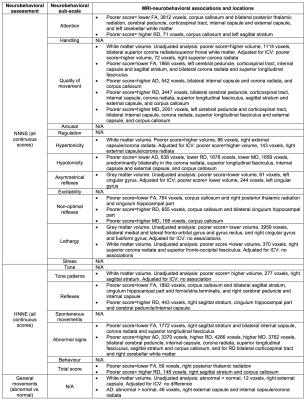
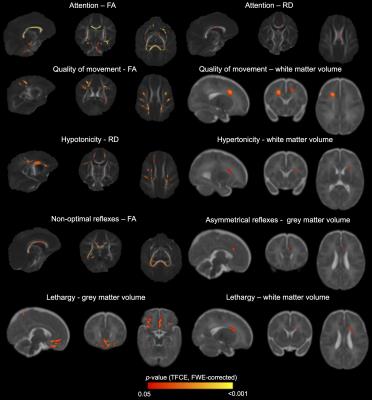
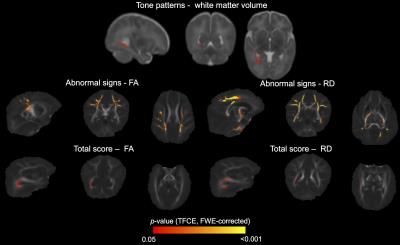
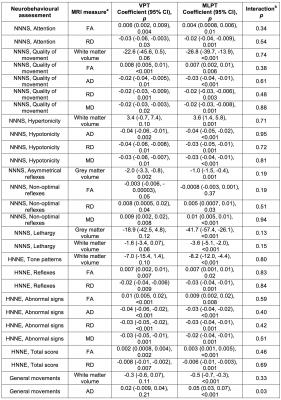
Many associations were identified between MRI measures and neurobehavioural outcomes. For the NNNS assessment, lower fractional anisotropy, higher diffusivities and/or lower grey and white matter volumes in the regions listed in Table 1 and shown in Figure 1 were associated with poorer scores for the sub-scales of attention, quality of movement, asymmetrical reflexes, non-optimal reflexes and lethargy. Additionally, lower diffusivities and/or higher white matter volumes were associated with poorer scores for quality of movement, hypotonicity and hypertonicity sub-scales (Table 1, Figure 1). For the HNNE, lower fractional anisotropy and/or higher diffusivities were associated with poorer scores for the sub-scales of reflexes and abnormal signs and the total score (Table 1, Figure 2). Additionally, higher white matter volume was associated with poorer scores for the tone patterns sub-scale (Table 1, Figure 2). Abnormal general movements were associated with lower white matter volume and higher diffusivity (Table 1). Some of the volume-behaviour relationships remained after adjusting for ICV, while some weakened after adjusting for ICV (Table 1). In general, findings were similar in VPT and MLPT infants, although some findings were stronger in MLPT infants, e.g. associations between MRI and general movements (Table 2).Discussion
Novel relationships were identified between brain structure in various regions and many aspects of neurobehaviour in preterm infants at TEA. The regions and tracts identified, and the directions of associations, differed depending on the neurobehavioural outcome, highlighting particular structure-function relationships. Regions commonly identified included the corpus callosum, sagittal stratum (including optic radiation), external capsule, motor fibres such as the internal capsule and corona radiata, and frontal and medial cortical regions.Conclusion
This study improves knowledge of brain-behaviour relationships early in life and the structural brain changes underlying neurobehavioural problems in preterm infants at TEA.Acknowledgements
No acknowledgement found.References
1. Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(Pt 3):667-677.
2. Brown NC, Doyle LW, Bear MJ, et al. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics. 2006;118(6):2461-2471.
3. Spittle AJ, Spencer-Smith MM, Cheong JLY, et al. General Movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics. 2013;132(2):e452-458.
4. Einspieler C, Prechtl HF, Bos AF, et al. Prechtl's method on the qualitative assessment of general Movements in preterm, term and young infants, Volume 167. London: Mac Keith Press; 2004.
5. Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;(113):641–667.
6. Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. J Pediatr. 1998;(133):406–416.
7. Beare RJ, Chen J, Kelly CE, et al. Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Front Neuroinform. 2016;10(12). doi: 10.3389/fninf.2016.00012.
8. Kuklisova-Murgasova M, Aljabar P, Srinivasan L, et al. A dynamic 4D probabilistic atlas of the developing brain. NeuroImage. 2011;54(4):2750-2763.
9. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487-1505.
10. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. NeuroImage. 2014;92:381-397.
11. Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. NeuroImage. 2011;56(1):8-20.
Figures