4104
Early life predictors of brain development at term-equivalent age in infants born across the gestational ages.1Murdoch Childrens Research Institute, Melbourne, Australia, 2Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 3Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 4Department of Medicine, Monash Medical Centre, Monash Univeristy, Melbourne, Australia, 5Royal Women’s Hospital, Melbourne, Australia, 6Department of Obstetrics and Gynaecology, The University of Melbourne, Melbourne, Australia, 7Department of Physiotherapy, The University of Melbourne, Melbourne, Australia
Synopsis
Many early life factors contribute to how well a preterm child will develop, and these affect the brain differently based on how early the infant is born. The current study found that earlier gestational age is related to smaller brain volumes and less mature white matter at term-equivalent age. Correlated with these brain measures were lower birthweight SD score, multiple birth or high social risk. We show that infants born moderate and late preterm have altered brain development, not just those born very preterm, and there is a differential effect of early life predictors based on gestational age.
Introduction
Very preterm (VP; born <32 weeks’ gestational age; GA) infants currently attract the most attention. However, infants born moderate (MP; 32-33 weeks’ GA) and late preterm (LP; 34-36 weeks’ GA) also have higher rates of impairment than children born full-term (FT; ≥37 weeks’ GA).1 Many perinatal predictors for brain abnormality in VP infants are known, but the risk factors associated with brain growth and development in MP and LP infants are currently understudied, and likely differ from those identified for VP infants. Aims: (1) Determine the effect of GA on brain volumes and white matter (WM) microstructure at term-equivalent age; (2) Investigate the effects of early life predictors on brain volumes and WM microstructure, and if these relationships differ according to GA.Methods
592 infants were recruited including VP (<30 weeks’ GA), MP, LP, and FT. T2-weighted (TR 8910ms, TE 152ms, 1mm3 isotropic voxels) and diffusion-weighted images (TR 20400ms, TE 120ms, 1.2mm3 isotropic voxels, 45 non-collinear gradient directions, b-values 100-1200 s/mm2) were acquired on a 3T Siemens scanner at term equivalent age (38-44 weeks’ GA). The automated Morphologically Adaptive Neonatal Tissue Segmentation (MANTiS) classified T2 images into WM and cortical grey matter (CGM).2 Voxel-based morphometry (VBM) was performed on CGM and WM tissue maps3 for 91 VP, 63 MP, 103 LP and 70 FT infants. Diffusion images were corrected for motion, eddy current,4 and echo planar image distortions. Fractional anisotropy (FA) and mean (MD), axial (AD) and radial diffusivity (RD) images were analyzed using Tract-Based Spatial Statistics (TBSS)5 for 92 VP, 69 MP, 120 LP and 80 FT infants. FSL’s Randomise was used with 1-factor 4-levels ANOVA models to test between GA groups, and post-hoc comparisons between GA increments (VP vs. MP, MP vs. LP, LP vs. FT). Perinatal variables (birthweight SD score, sex, multiple births, early postnatal growth, social risk) were correlated with volume and diffusion maps, with post-hoc tests to determine GA group differences. GA at MRI was adjusted for, and additionally intracranial volume for VBM analyses.Results
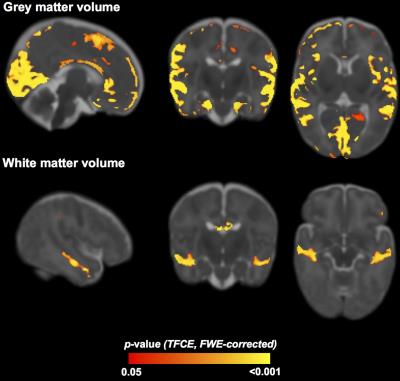
GA group was associated with regions making up 28% of the total CGM volume, including occipital, temporal, parietal and frontal lobes, and 3% of the WM volume, including the temporal lobe and corpus callosum (Figure 1). The main differences were between the VP vs. MP groups, with higher volume for the MP group in frontal lobe CGM and temporal lobe WM. There was also a small region in the corpus callosum that had lower volume in the LP vs. FT group.
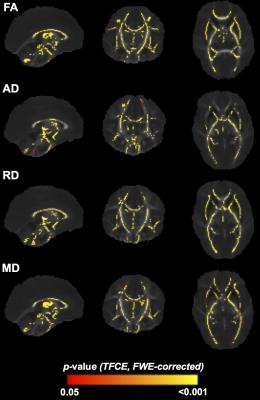
GA group had a significant association with FA (67% of the white matter skeleton), AD (45%), RD (59%) and MD (53%), located in most major WM tracts (Figure 2). While there were some regions of lower FA and higher RD and MD in the VP vs. MP infants (particularly corpus callosum), WM microstructural differences were more evident between LP and FT groups, with lower FA and higher RD, MD and AD in the LP group, located in corpus callosum, motor and visual tracts. The opposite pattern was seen for some regions within cerebellar WM.
Birthweight SD score was positively associated with CGM and WM volume in much of the brain for all GA groups, but diminished substantially after adjusting for intracranial volume. Birthweight SD score was also positively associated with FA and negatively with MD, RD and AD in many major tracts, driven mainly by the MP and LP groups.
CGM and WM volumes were higher in males than females, particularly for the MP and FT groups, but this diminished substantially after adjusting for intracranial volume. MD, RD and AD were higher in the internal and external capsule and optic radiations in males than females, driven mainly by the LP and FT groups.
Multiple birth was associated with lower frontal CGM and corpus callosum volume than single birth, particularly for the MP group.
Frontal-parietal CGM and WM and temporal WM volume were reduced for high vs. low social risk, particularly for LP and FT groups, but this disappeared after adjusting for intracranial volume.
Discussion
Earlier GA is related to smaller brain volumes and less mature WM at term-equivalent age, with corpus callosum and temporal lobe particularly affected. This study provides further evidence that brain vulnerabilities are not restricted to VP infants. Early life predictors that alter or delay brain development include lower birthweight SD score, multiple birth or high social risk, with a differential effect based on GA.Conclusion
This study helps determine the underlying causes and mechanisms for brain abnormalities in infants at risk of adverse outcomes.Acknowledgements
No acknowledgement found.References
1. Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3):e622-629.
2. Beare R, Chen J, Kelly CE, et al. Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Front Neuroinform. 2016;10(12). doi: 10.3389/fninf.2016.00012.
3. Kuklisova-Murgasova M, Aljabar P, Srinivasan L, et al. A dynamic 4D probabilistic atlas of the developing brain. NeuroImage. 2011;54(4):2750-2763.
4. Jenkinson M and Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143-156.
5. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487-1505.
Figures