4102
Convolutional Neural Networks for Identifying Preterm Infants at High Risk of Developmental Disorders1University of Edinburgh, Edinburgh, United Kingdom
Synopsis
Preterm birth is a major cause of neuropsychiatric impairment in childhood and leads to significant long-term clinical, educational and social problems. A major issue confronting clinicians who work with preterm infants and their families is the identification of infants who are most at risk for subsequent neurodevelopmental disability and who may benefit from early intervention services. We designed a system for the identification of preterm infants at high risk of developmental disorders using convolutional neural networks (CNN). The designed network yields an accuracy of 83.33%
INTRODUCTION
Early prediction of preterm infants at high risk of developmental disorders has the potential to improve the care for preterm infants and their families, and to stratify patient groups who may benefit from neuroproetctive therapies1. Currently, the early assessment of brain development in preterm infants and prognosis of outcome is heavily dependent on a subjective assessment of clinical and relatively low resolution imaging data2,3. The aim of this work is the creation of a system using convolutional neural networks (CNN)4 that enable the early detection preterm infants at high risk of developmental disorders based on high-resolution magnetic resonance imaging (MRI) information.METHODS
Participants: 32 preterm infants born at 28 weeks or less (mean PMA at birth 27.11 weeks, range 23.28-28.43 weeks) underwent brain MRI at term equivalent age (mean PMA 39.97 weeks, range 38-44.29 weeks). MRI data were also acquired from 28 healthy infants born at full term (mean PMA 39.68 weeks, range 37.28-41.14 weeks) underwent brain MRI at mean age 41.88 weeks. Infants were examined in natural sleep with pulse oximetry, temperature and electrocardiography data monitoring. Ear protection was used for each infant, comprising earplugs placed in the external ear and neonatal earmuffs (MiniMuffs, Natus Medical Inc., CA). Ethical approval was granted by the National Research Ethics Service (South East Scotland Research Ethics Committee) and NHS Research and Development, and informed written parental consent was obtained. MRI acquisition: A Siemens MAGNETOM Verio 3T MRI clinical scanner was used to acquire 3D T1-weighted (T1w) MPRAGE data with voxel size = 1 × 1 × 1 mm3. Image Processing: The brain was extracted from T1w images (by removing non-brain tissues and skull) using ALFA5, corrected for intensity inhomogeneity using N46, linearly aligned to a common template space7, and rescaled to a range of 0 to 255. Then, images were resampled to a voxel size of 3 × 3 × 3 mm3, and a Gaussian filter with 2 × 2 × 2 convolutional kernel was applied. We used these processed images as an input for the CNN. Network architecture: A CNN with multiple convolution layers was used; a schematic of the network is shown in Fig. 1. In the first convolutional layer, 16 filters with a kernel size of 5 × 5 × 5 voxels were used, while the second convolutional layer had 32 filters with a kernel size of 3 × 3 × 3 voxels. Each convolutional layer was followed by Rectified Linear Unit (ReLU) activation layer. Max-pooling with a kernel size of 2 × 2 × 2 voxels was applied to the convolved images. The output of the max-pooling layer was input to a fully connected layer with 128 nodes. These nodes are subsequently connected to the softmax output layer with 2 nodes (healthy or high-risk infant). The network was regularised using Dropout8 after the max-pooling and fully connected layers with a rate of 50%. Evaluation: The images from the dataset (N=60) were split into: training (48 images) and test (12 images). Then, the training data was split further into: 40 images for training, and 8 images for validation and parameter estimation. Multiple epochs were used in the training process, where typical number of epochs used = 10.RESULTS
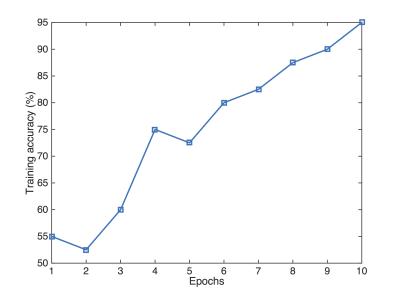
The performance of the CNN on the training images for each epoch is shown in Fig. 2. Our network achieved a mean training accuracy of 95%, and 88.75% mean accuracy for validation. The network achieved an accuracy of 83.33% for the test data.DISCUSSION AND CONCLUSION
We present a classification system based on convolutional neural networks. The network exhibits high accuracy, with capabilities for identifying preterm infants at high risk of developmental disorders using structural magnetic resonance images. Our network is trained by 3D brain volumes in less than 30 seconds, making it applicable to the automated analysis of larger study cohorts.Acknowledgements
We are grateful to the families who consented to take part in the study and to the nursing and radiography staff at the Clinical Research Imaging Centre, University of Edinburgh (http://www.cric.ed.ac.uk) who participated in scanning the infants. The study was supported by Theirworld, NHS Research Scotland, and NHS Lothian Research and Development. This work was undertaken in the MRC Centre for Reproductive Health which is funded by the MRC Centre grant MR/N022556/1.References
1. Abbott, A., Neuroscience: The brain, interrupted. Nature, 2015. 518(7537): p. 24-6.
2. Brossard-Racine, M., A.J. du Plessis, and C. Limperopoulos, Developmental Cerebellar Cognitive Affective Syndrome in Ex-preterm Survivors Following Cerebellar Injury. Cerebellum, 2015. 14(2): p. 151-164.
3. Iwata, S., et al., Qualitative Brain MRI at Term and Cognitive Outcomes at 9 Years After Very Preterm Birth. Pediatrics, 2012. 129(5): p. E1138-E1147.
4. LeCun, Y., Y. Bengio, and G. Hinton, Deep learning. Nature, 2015. 521(7553): p. 436-444.
5. Serag, A., et al., Accurate Learning with Few Atlases (ALFA): an algorithm for MRI neonatal brain extraction and comparison with 11 publicly available methods. Scientific Reports, 2016. 6.
6. Tustison, N.J., et al., N4ITK: Improved N3 Bias Correction. Medical Imaging, IEEE Transactions on, 2010. 29(6): p. 1310-1320.
7. Serag, A., et al., Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage, 2012. 59(3): p. 2255-65.
8. Srivastava, N., et al., Dropout: A Simple Way to Prevent Neural Networks from Overfitting. Journal of Machine Learning Research, 2014. 15: p. 1929-1958.
Figures