4101
Characterization of Extensive Microstructural Variations Associated with Punctate White Matter Lesions in Preterm Neonates1Department of Radiology, the First Affiliated Hospital, Xi'an Jiaotong University, Xi'an, People's Republic of China, 2Department of Biomedical Engineering, the Key Laboratory of Biomedical Information Engineering of the Ministry of Education, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, People's Republic of China, 3Department of Diagnostic Radiology, the University of Hong Kong, Hong Kong, People's Republic of China, 4MR Research China, GE Healthcare, Bei Jing, People's Republic of China
Synopsis
Punctate white matter lesions (PWML) are common in preterm neonates. Neurodevelopmental outcome of the neonates is related to the lesion extensions. This study aimed to characterize the extent of microstructural variations for different PWML grades. White matter microstructural variations were different across PWML grades. The severe PWML were associated with extensive microstructural changes. Pattern of increased AD, increased RD, and reduced/unchanged FA was found proximal to the PWML. Unchanged AD, increased RD, and reduced/unchanged FA were observed in the vast regions distal from the PWML. These findings may help in determining outcomes of PWML and treatment strategies.
INTRODUCTION
Punctate white matter lesions (PWML) are common in neonates and have been found in more than 20% of preterm neonates 1, 2. PWML can be identified on conventional MRI as hyperintensity on T1WI and hypointensity on T2WI 3, 4. These lesions may cause severe neurological disorders, such as cerebral palsy 2. The neurodevelopmental outcome is related to lesion extensions 5. This study aimed to characterize the extent of microstructural variations for different PWML grades.METHODS
This study was approved by the local Institutional Review Board. Before the MRI scan, parents of neonates were informed about the goal and risks involved in the MR scan. Written informed consents were obtained. Inclusion criterion was the evidence of punctate lesions in the cerebral white matter, which presented on T1WI and T2WI. Subjects with clinical diagnosis of congenital malformations of the central nervous system, infections, metabolic disorders, hydrocephalus, gray matter lesions or major destructive white matter lesions were excluded. Controls were matched for gender, gestational age (GA), postnatal age at MRI scan, and birth weight and did not have any MRI abnormality or evidence of a clinical episode that might cause cerebral damages.
The MRI datasets used in this study were acquired for clinical examination and diagnosis. To reduce head movement and complete the MRI procedure, the patients were sedated with a relatively low dose of oral chloral hydrate (25−50 mg/kg). The patient selection, monitoring, and management were performed following the guidelines. Three-dimensional FSPGR T1WI, FSE T2WI and single-shot EPI diffusion tensor imaging (DTI) were performed on a 3.0T scanner (General Electric Signa HDXT, WI, USA) with an eight-channel head coil. The other parameters for DTI were: 35 gradient directions; b values=0 and 1000 s/mm2; TR/TE=5500/95−105 ms; slice thickness=4 mm; FOV=180×180 mm2; and matrix size=128×128. Neonates with PWML were divided into 3 grades (from mild to severe: grade I−III) 3. Fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) between PWML and controls were compared using tract-based spatial statistics and tract quantification methods. Wilcoxon signed ranks test was used to evaluate differences in GA, birth weight, postnatal ages at MRI scan, postmenstrual age (PMA), and regional values of DTI metrics between PWML and control groups. Tests were considered significant at P<0.05.
RESULTS
A total of 33 preterm neonates with PWML and 33 matched controls were enrolled. The patient numbers in grades I, II, and III were 15, 9, and 9, respectively. No significant differences in GA, postnatal ages at MRI scan, PMA, or birth weight were found between neonates with PWML and controls (Table 1).
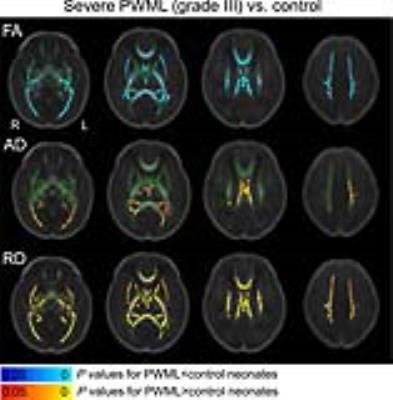
Extensive microstructural changes were observed in neonates with PWML of grade III (Figure 1), while no significant changes in DTI metrics were found for grades I and II. Pattern of increased AD, increased RD, and reduced/unchanged FA was found proximal to the PWML in the centrum semiovale and/or corona radiata, the trigone of the lateral ventricles, splenium of corpus callosum (SCC) and optic radiations (OR). Unchanged AD, increased RD, and reduced/unchanged FA were observed in the vast regions distal from the PWML (Figure 2).
DISCUSSION
Though the etiology and pathogenesis are nonspecific and complex 6, the location of PWML is regular. PWML were common along the corona radiata, in the posterior periventricular white matter, and along the OR. The distribution of injury is in agreement with areas of high microglia density and deep medullary venous anatomy 5. The results revealed that the extent of microstructural changes were different across PWML grades. This may be due to the different pathophysiological mechanisms or the different effects on brain development. In this study, severe PWML related alterations were widespread along specific white matter tracts. The alterations in projection, commissural, and association fibers may result in motor, sensory, and cognitive disorders. Changes in DTI metrics demonstrated a PWML site-related spatial distribution. These two patterns may relate to different mechanisms underlying the effects of PWML on brain development, including the delayed oligodendrocyte proliferation or the death of oligodendrocyte progenitors; and the disturbed maturation of oligodendrocytes.CONCLUSION
White matter microstructural variations were different across PWML grades. The severe PWML were associated with extensive microstructural changes. The patterns of changes were related to regional distance to the PWML. These findings may help in determining outcomes of PWML and treatment strategies.Acknowledgements
This work was supported by the grant from the National Natural Science Foundation of China (No.81171317, 81471631), the National Key Research and Development Program of China (2016YFC0100300), and the 2011 New Century Excellent Talent Support Plan from the Ministry of Education of China (NCET-11-0438).References
1. Bassi L, Chew A, Merchant N, et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res 2011;69:561-566.
2. de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 2011;261:899-906.
3. Childs AM, Cornette L, Ramenghi LA, et al. Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol 2001;56:647-655.
4. Tortora D, Panara V, Mattei PA, et al. Comparing 3T T1-weighted sequences in identifying hyperintense punctate lesions in preterm neonates. AJNR Am J Neuroradiol 2015;36:581-586.
5. Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M. The premature brain: developmental and lesional anatomy. Neuroradiology 2013;55:23-40.
6. Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010;52:505-521.
Figures