4099
White Matter Brain Asymmetries in Preterm and at Term NewbornsPaola Scifo1,2, Pasquale Anthony Della Rosa2,3, Elisa Marchetta2,3, Silvia Pontesilli2,3, Roberta Scotti2,3, Antonella Poloniato4, Andrea Falini2,3, and Cristina Baldoli2,3
1Nuclear Medicine Dept, San Raffale Scientific Institute, Milan, Italy, 2CERMAC, San Raffaele University, Milan, Italy, 3Neuroradiology Dept, San Raffaele Scientific Institute, Milan, Italy, 4Pediatrics Dept, San Raffaele Scientific Institute, Milan, Italy
Synopsis
Aim of this study is to investigate, through DTI, structural white matter (WM) asymmetries in newborns and compare these differences in control and in preterm newborns scanned at term. Both controls and preterms show multiple regions of asymmetries in all the DTI indices. A small area localised in the talamic radiations was found to have a significant difference of WM brain asymmetries between the two groups of subjects.
INTRODUCTION
In Neuroscience, the lateralization of some cognitive and motor functions is an important issue often related to asymmetries of brain structures1. Many studies have been focused on newborns’s brain to understand whether these asymmetries are already present early in life or if they develop together with functional lateralization and connectivity2,3. Preterm studies offer insights into antenatal brain developmental phases4. In a previous work5, we have found longitudinal modifications in the lateralization of functional activations related to language during the first two months of life of preterm newborns. The comparison between control subjects and preterm newborns at term revealed differences in these activations in posterior thalamic radiations. Aim of this study is to investigate, through DTI, structural white matter (WM) asymmetries in newborns and compare these differences (Left vs Right - LvsR) in control and in preterm newborns scanned at term.METHODS
Two groups of newborns were studied: a) 29 control subjects, born at term; b) 24 preterm newborns (born within 24-33 weeks of post menstrual age (PMA)), without any evident WM injuries on conventional MR examinations. MR acquisitions were performed for normal subjects within the first 4 days of life, while preterm infants were scanned at term equivalent age (40th week PMA). They were acquired on a 3T scanner (Philips, Intera) and included a clinical examination (T1, T2) and DTI acquisitions (DW-EPI, 21 directions for diffusion gradients, b-value=700s/mm2, voxel size=2x2x3.5mm3). During the examinations, infants were spontaneously asleep without sedation. DTI maps were created using FDT diffusion toolbox6: eddy current distortions were corrected and binary masked of the brain tissue were generated with BET software on the b=0 images. Tensor maps for FA, MD; L1, L2, L3 were calculated with DITfit. Analyses were performed optimising the preprocessing steps for newborn images7. Mean image and skeletons were derived after a non linear transformation, setting the subject best representing the group as target. Finally, hemispheric developmental asymmetries were measured, using 1) tbss_sym to create a symmetric skeleton and difference images between L and R hemispheres and 2) randomise for including the DTI indices for both Left (LH) and right hemispheres (RH) in a two-sample t-test comparing the tract asymme tries between the two groups (controls and preterms).RESULTS
TBSS analyses of DTI indices revealed significant WM asymmetries in FA. Both in controls and preterm newborns higher FA in the LH than in the RH was found in deeper areas of WM and in optic radiations; while lower FA in LH were found in more peripheral areas (see fig.1, fig.2). A small area of significant difference between the two groups of subjects emerged in the fibers of thalamic radiations, where the left-laterality was higher in controls than in preterm newborns (see fig.3). L1 had similar results as FA while MD, L2 and L3 analyses gave similar but inverse results: the higher diffusivity indices in LH than RH were localized in more peripheral areas while the lower MD in LH than in RH were localized in deeper WM.DISCUSSION
These data confirm, in part, results of in previous studies2,4 related to the early asymmetries of WM brain structures, obtained with different methods of analysis. Our results might suggest that both in controls and in preterm newborns the LH is more “specialized” or “mature” than the RH in deep regions while the RH is more “specialized” or “mature” than the LH in more peripheral areas. The difference between the two groups seems to confirm previous results5, that there are some kind of modifications in the development of thalamic radiations in preterm newborns with respect to controls. This might be related to alterations in the development of the transient subplate structural connectivity during the third trimester of gestation when premature birth takes place.CONCLUSIONS
This study has shown that brain structure asymmetries are present both in control and in preterm newborns at term in deep and peripheral areas. Asymmetries in maturation indices are higher in controls than in preterm newborns in thalamic radiations. These results might be related to modifications in the development of subplate structural connectivity.Acknowledgements
No acknowledgement found.References
1. Toga, AW, Nat. Rev. Neurosci; 2003; 4:37–48
2. Dubois J, Cerebral Cortex; 2009;19:414—423
3. Ratnarajah N, NeuroImage; 2013, 75:187–194
4. Liu Y, NeuroImage; 2010; 51: 783–788
5. Baldoli C; Brain Struct Funct. 2015; 220(6):3733-51
6. FSL, www.fmrib.ox.ac.uk/fsl/
7. Ball G, Neuroimage; 2010; 53: 94-102
Figures
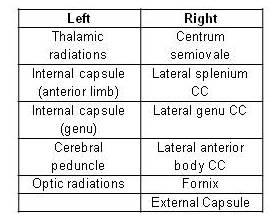
Fig.1: Significant (p<0.05
corr) asymmetric WM areas with higher FA than in the opposite hemisphere
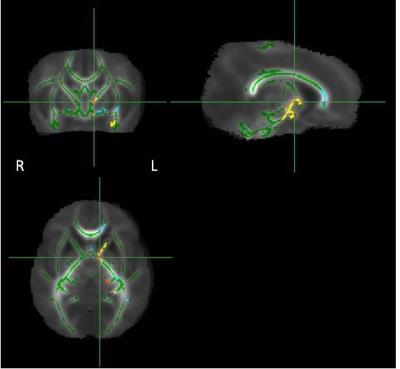
Fig.2: WM areas where Controls have
asymmetries in FA: Yellow-red in Left>Right, Light-blue in Right>Left
(p<0.05 corrected). All the differences have been represented in the Left side of the brain.
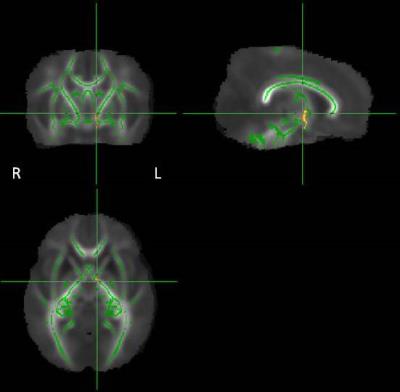
Fig.3: WM areas where Controls have
higher asymmetries in FA than Preterms (p<0.05
corrected). All
the
differences have been represented in the Left
side of the brain.