4097
Patterns of Microstructural Correlations in the White Matter of the Neonatal Brain1Waisman Center, University of Wisconsin-Madison, Madison, WI, United States, 2Center for Healthy Minds, University of Wisconsin-Madison, Madison, WI, United States, 3Psychology, University of Wisconsin-Madison, Madison, WI, United States, 4Psychiatry, University of Wisconsin-Madison, Madison, WI, United States, 5Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Quantitative MRI affords a unique opportunity to map dynamic patterns of neurodevelopment and provide insight into relations between brain maturation and emerging cognition. Using a data-driven technique, we investigated underlying patterns of white matter microstructure and subsequently examined the relationships of these microstructural correlations with respect to gestation-corrected age. We demonstrate that patterns of white matter development may be informative to studies of early brain development.
Introduction
Early in life, the brain establishes neural circuitry necessary for the development of behavior and cognition (Casey et al., 2005). Advances in behavior and cognition are facilitated by the formation of complex neural networks able to communicate via white matter microstructure (Fields, 2008). These brain networks are important to normative brain connectivity and function (Durston and Casey, 2006). Thus, garnering a better understanding of factors that may influence the formation of these networks during the earliest stages of life is of critical importance. Diffusion imaging, a technique used to assess the white matter microstructure, has been influential to the study of white matter development. Moreover, as distinct white matter regions work harmoniously with others, an informative approach to white matter development will be to study underlying patterns and characteristics that can be detected through multivariate and machine learning techniques. Here, we utilized a data-driven technique to identify developing microstructural patterns of white matter in a large sample of one-month infants and subsequently examined the associations of these microstructural patterns with gestation-corrected age.Materials/Methods
Participating mothers were identified during the second trimester of pregnancy. When infants were one month old, they underwent non-sedated magnetic resonance imaging (MRI) in which multi-shell diffusion imaging data were acquired. Diffusion weighted images underwent standard processing, including correction of motion and eddy currents, non-parenchyma tissue removal, and fitting of the diffusion tensor using RESTORE (Chang et al., 2005). Additional microstructural data were constructed by fitting acquired diffusion imaging data to the neurite orientation dispersion and density imaging (NODDI) model (Zhang et al., 2012). Fractional anisotropy (FA), and mean, radial, and axial diffusivity (MD, RD, AD, respectively), as well as NODDI parameter maps, including the intra-cellular and free water fractions (vIC, vISO ) and orientation dispersion index (ODI) were calculated and aligned to a study-specific template created using DTI-TK. Spatially aligned parameter maps were smoothed with a 5mm full-width-at-half-maximum kernel and concatenated into a single 4D image file. Spatial independent component analysis (sICA) was performed using the MELODIC tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC), providing spatially independent white matter networks. Associations of these microstructural components were constructed by extracting the mean parameter value from each component and examining the association with gestation-corrected age.Results
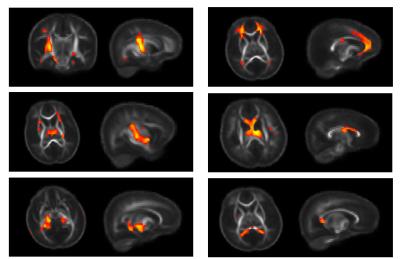
Multi-shell diffusion imaging data from 95 one-month-old infants
were successfully acquired. sICA identified 26 spatially independent components,
with 21 of these corresponding to biologically relevant bilateral and
unilateral brain areas, including regions such as the brain stem, internal
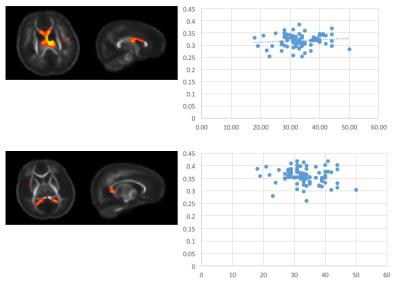
capsules, cingulum, and corpus callosum. Differential patterns of development appear
across components, with differing components having dissimilar developmental
trends, such as distinct developmental slopes and/or intercepts. Such findings
suggest sICA is both sensitive to the microstructural changes in the brain and that
these microstructural components have differential associations with
gestational age. Conclusion
Measures derived from diffusion imaging combined with data-driven
analysis techniques such as sICA can be used to parcel the brain into biologically
meaningful components. Identified patterns additionally appear to undergo rapid
and dynamic patterns of development. These findings provide an important step
for understanding developmental patterns of white matter microstructure. The functional role of these networks as the
infant matures will be investigated in the future by examining associations
with specific behavioral outcomes. Future research will also explore the
utility of these early white matter components as markers of socio-emotional and
cognitive/behavioral outcomes in later infancy and toddlerhood. Acknowledgements
We thank the many families that participated in this research.References
Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 2005; 9: 104–110.
Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences 2008; 31: 361–370.
Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia 2006; 44: 2149–2157.
Chang LC, Jones DK, Pierpaoli C. RESTORE: Robust estimation of tensors by outlier rejection. Magn Reson Med 2005; 53: 1088–1095.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012; 61: 1000–1016.
Figures