4093
Hyperpolarized Water Perfusion in the Porcine Brain – a Pilot Study1The MR Research Centre, Aarhus University, 8200, Denmark, 2Danish Diabetes Academy, Odense, Denmark, 3Department of Electrical Engineering, Technical University of Denmark, 4Department of Cardiology, Aarhus University Hospital, Denmark, 5GE Healthcare, Denmark
Synopsis
Dynamic Contrast-Enhanced MR (DCE-MR) perfusion assessment with gadolinium contrast agents is currently the most widely used cerebral perfusion MR method. Hyperpolarized water has recently been shown to succeed 13C probes as angiography probe. In this study, we demonstrate the feasibility of hyperpolarized water for visualizing the brain vasculature of a large animal in a clinically relevant setting. In detail, reference perfusion values were obtained and large to small arteries could be identified.
Introduction
Dynamic Contrast-Enhanced MR (DCE-MR) perfusion assessment with gadolinium contrast agents is currently the most widely used cerebral perfusion MR method1. The method relies on the acquisition of a dynamic series of T1-weighted images during administration of the contrast agent, and as such, the contrast is converted to an apparent concentration, which is then used to model the cerebral perfusion2. However, increasing awareness on the deposition of gadolinium contrast in brain tissue supports the pursuit of novel perfusion measures such as arterial spin labelling (ASL). ASL is, however, inherently hampered by the low signal to noise ratio. In contrary, hyperpolarized MR is the signal source in itself and thus simplifies the quantification3. A limitation, however, is the need for 13C-molecular contrast agents, which limits spatial and temporal resolutions significantly. Recently, hyperpolarized water has been demonstrated in rodents4,5 and in the porcine kidney6, showing superior spatial resolution compared to 13C angiographies. Here we investigated the potential for utilizing hyperpolarized water in the porcine brain, in conjunction with a model-free deconvolution, to map the cerebral perfusion7.Methods
A healthy female Danish domestic pig weighing 31 kg was anaesthetized via continuous intravenous infusion of both Propofol and Fentanyl. Catheterizations were performed in the left femoral vein for blood sampling and the right and left femoral arteries for administration of hyperpolarized water, Percutaneous Intervention (PCI) -procedure and invasive blood pressure monitoring. 15 mL hyperpolarized water was injected over 5 s, initiated approximately 22 s after dissolution. A sample of 1 mL 30 mM TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl, 98%) in H2O/glycerol 1:1 (w/w) was prepared. Dissolution medium consisted of D2O with 1 mM calcium disodium ethylenediaminetetraacetic acid (EDTA) and 9 g/L NaCl (all from Sigma Aldrich, Denmark). The sample vial was rapidly transferred from the liquid nitrogen bath to the 5 T magnet in the polarizer (SPINlab, GE Healthcare, Denmark). The sample was irradiated with app. 50 mW microwaves at 139.923 GHz for one hour. After dissolution, the radical was removed by extraction in 25 mL heptane (Sigma Aldrich, Denmark) in a separatory funnel. MRI imaging was performed on a 3 T GE HDx with an 8-channel cardiac array receiver coil (GE Healthcare, USA). Axial and coronal images were acquired to cover the brain to allow for planning of the angiography slab for hyperpolarized water imaging. The sequence was as follows: a 3D T1 weighted sequence with a standard SSFP (TE = 1.1 ms, TR = 2.7 ms, FA = 35°, matrix 256x256, FOV = 340x340 mm2, in-plane resolution of 1.3 mm and slice thickness of 3 mm). Angiographies were acquired in a coronal plane covering the brain using a gradient echo sequence: FA = 5°, slice thickness of 40 mm, TR = 3.4 ms, TE = 0.984 ms, matrix 256x256 and FOV = 140x140 mm2. The acquisition time for each frame was 870 ms and 60 frames were acquired. Brain perfusion maps were calculated in OsiriX (Pixmeo, Geneva, Switzerland) using an open source OsiriX plug-in for T1-DCE-MRI perfusion analysis8. A pixel-by-pixel model-free, fast deconvolution was applied, assuming a direct signal enhancement of the hyperpolarized water, a hematocrit of 0.45 and a regularization of 0.15. Signal-to-Noise-Ratio (SNR) was measure as peak signal versus baseline noise in the input ROI.Results
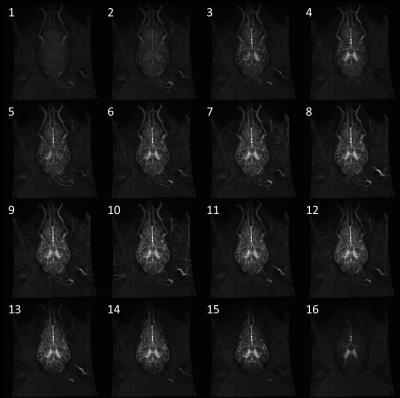
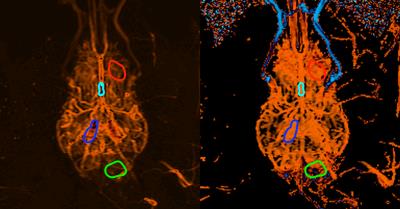
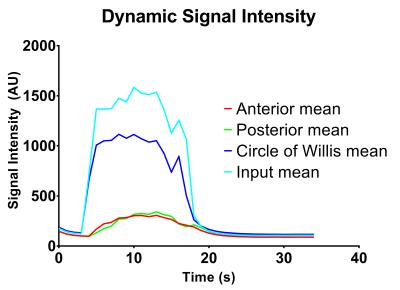
Injection of hyperpolarized water into the brain allowed for visualization of both large and small vessel as well as for acquisition of perfusion values from brain tissue. Figure 1 shows the acquired frames and presents the amount of details from vasculature we have obtained with this setup. The calculated perfusion and MTT maps are shown in Figure 2. The results from the perfusion ROI analysis are (ROI mean value): Anterior, 255 (mL/100mL/min); Posterior, 220 (mL/100mL/min); Circle of Willis, 1895 (mL/100mL/min); Input, 2645 (mL/100mL/min). The results from the MTT ROI analysis are (ROI mean value): Anterior, 1.09 (s); Posterior, 1.77 (s); Circle of Willis, 0.80 (s); Input, 1.00 (s). Figure 3 shows the dynamic evolution of hyperpolarized water entering the four ROIs and the difference between peak signal from input to anterior and posterior part of the brain is clearly observed. SNR was measured to be 273.Conclusion
In this study, we demonstrate the feasibility of hyperpolarized water for visualizing the brain vasculature of a large animal in a clinically relevant setting. Furthermore, perfusion values within normal physiological range are obtained from the dynamic acquisition. We believe this to be a promising case for further sequence and method optimization. We expect that the polarization of the hyperpolarized water can be increased by a factor 5-10 relative to the current study.Acknowledgements
Funded by The Danish Diabetes Academy supported by the Novo Nordisk Foundation.References
1. Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: The Five Most Frequently Asked Technical Questions. AJR. American journal of roentgenology. 2013;200(1):24-34.
2. Khalifa F, Soliman A, El-Baz A, et al. Models and methods for analyzing DCE-MRI: A review. Medical Physics. 2014;41(12):124301.
3. Johansson E, Mansson S, Wirestam R, et al. Cerebral perfusion assessment by bolus tracking using hyperpolarized 13C. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;51(3):464-472.
4. Lingwood MD, Siaw TA, Sailasuta N, et al. Hyperpolarized Water as an MR Imaging Contrast Agent: Feasibility of in Vivo Imaging in a Rat Model. Radiology. 2012;265(2):418-425.
5. Ardenkjaer-Larsen JH, Laustsen C, Bowen S, Rizi R. Hyperpolarized H2O MR angiography. Magnetic Resonance in Medicine. 2014;71(1):50-56.
6. Wigh Lipso K, Hansen ES, Tougaard RS, et al. Renal MR angiography and perfusion in the pig using hyperpolarized water. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2016.
7. Østergaard L, Sorensen AG, Kwong KK, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magnetic Resonance in Medicine. 1996;36(5):726-736.
8. Zollner FG, Weisser G, Reich M, et al. UMMPerfusion: an open source software tool towards quantitative MRI perfusion analysis in clinical routine. J Digit Imaging. 2013;26(2):344-352.
Figures