4091
Brain-wide functional connectivity changes following self-administration of cocaine and a period of abstinence1Neuroscience curriculum, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Biomedical Research Imaging Center (BRIC), University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Neurology, Chapel Hill, NC, United States, 5Radiology, Chapel Hill, NC, United States, 6Psychology, University of North Carolina at Chapel Hill, NC, United States, 7Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 8Biomedical Engineering, Chapel Hill, NC, United States, 9Cell Biology and Physiology, Chapel Hill, NC, United States
Synopsis
Several different neural systems are likely to be dysregulated to promote maladaptive behaviors associated with drug addiction. Here, we investigate changes in global functional connectivity across the brain following self-administration of cocaine and a period of abstinence in rats. We found whole brain enhancement in synchronized activity immediately following cocaine self-administration compared to pre-cocaine. Furthermore, whole brain network connectivity continued to strengthen following a period of abstinence. These data suggest that the dynamic shifts in functional connectivity following cocaine exposure persist during periods of abstinence and may provide a critical mechanistic link to relapse susceptibility.
Purpose
Addiction is a chronically relapsing disease characterized by compulsive drug-seeking and drug-taking despite severe adverse consequences. All drugs of abuse exert their reinforcing effects, at least in part, through the mesolimbic dopamine system1. Although the direct consequences of dopamine signaling are largely restricted to brain regions within this pathway, we recently found that selective activation of ventral tegmental area (VTA) DA neurons recruits several additional anatomically distinct regions throughout the brain, not typically associated with dopamine release events2. Considering this, it is possible that drug exposure in addiction causes long-lasting adaptations not only in dopamine-related circuits, but on a global scale. To investigate this, we evaluated changes in global functional connectivity across the brain following self-administration of cocaine and a period of abstinence. We hypothesized that cocaine exposure does not only alter functional connectivity within the mesolimbic dopaminergic system, but will also result in whole brain network remodeling.Methods
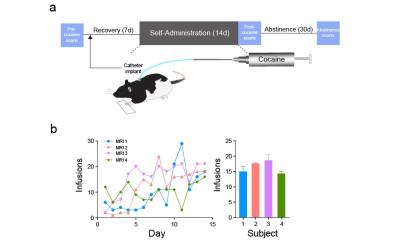
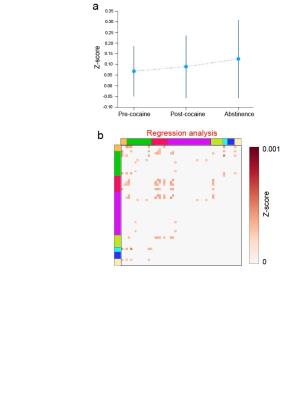
Adult (400–450 g) male tyrosine hydroxylase (TH)-ires-cre negative Long-Evans rats (n=4) underwent 6 consecutive 5-minute resting state functional connectivity MRI (fcMRI) scans prior to cocaine self-administration (pre-cocaine), immediately following cocaine self-administration (post-cocaine) and after 30d of abstinence. Following the pre-cocaine fcMRI scans, rats were surgically implanted with interjugular catheters and trained to self-administer cocaine during daily 2h sessions for 14 days. Lever pressing resulted in intravenous cocaine delivery (0.33 mg/infusion) via a computer-controlled syringe pump. On the day of fcMRI experiments, each rat was endotracheally intubated, ventilated, and sedated using dexmedetomidine (0.1 mg/kg/hr) and pancuronium bromide (1.0 mg/kg/hr) with 0.5% isoflurane. Single shot gradient echo-EPI sequence (spectral width=300 kHz, TR/TE=1000/13 ms, FOV=2.56x2.56 cm2, slice thickness= 1 mm, matrix=80x80) were acquired using a 9.4T MR scanner and a custom-built surface coil. We a priori defined 42 anatomical subregions across our imaging slices and further clustered these regions into established rodent brain networks2,3. Correlation matrices were constructed based on Pearson correlation coefficient for averaged data (6 repeated 5-min scans) for each animal per condition. After Fisher’s z-transformation, a one-sample t-test was conducted for each pairwise connection and significant connections were defined as p < 0.01. Pre-cocaine, post-cocaine, and abstinence matrices were then averaged across animals. A least squares linear regression was performed to fit the global average trend across conditions (Fig. 3) to determine which connections demonstrated a strengthening trend that was consistent with our hypothesis model.Results
Schematic of experimental design is shown in Fig. 1a. Rates of self-administration of cocaine were
comparable between subjects (Fig. 1b). Across 42 brain nuclei seed regions within
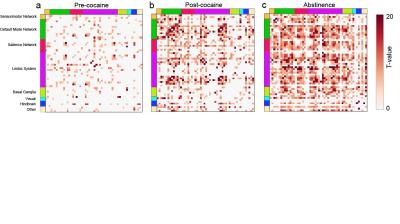
defined brain networks, functional connectivity analysis revealed a global
enhancement in synchronized activity between brain structures in the period
immediately following cocaine self-administration compared to pre-cocaine (Fig.
2a,b). This strengthening in global functional connectivity following
cocaine exposure was persistent and enhanced following a period of abstinence (Fig. 2c). ROI pairs that displayed an enhancement of
functional connectivity over the course of the experiment, and thus consistent
with our hypothesis model (Fig. 3a)
are shown in Fig. 3b.Discussion
Data has shown that pathologic forms of neuroplasticity underlie addiction, leading to high relapse susceptibility even following long periods of abstinence4. In fact, throughout abstinence drug cravings and the associated neuropathology becomes more pronounced, a process known as incubation5. Here, we extend those findings to show that cocaine self-administration orchestrates dynamic shifts in functional connectivity across many anatomically defined neuronal network nodes, such that the drug-evoked functional connectivity modifications continue to evolve long after the onset of drug abstinence.Conclusion
These data suggest that cocaine exposure may degrade optimal neuronal network dynamics, which in turn may shift brain activity to promote maladaptive states as seen in addiction. This research provides a means to identify novel neuroanatomical circuit nodes associated with phases of addiction, and for future experiments to explore whether network-based interventions (i.e. deep brain stimulation (DBS), transcranial current brain stimulation (tCS)) can normalize maladaptive network activity.Acknowledgements
We thank members of Stuber, Shih and Carelli laboratories for discussion. HKD was supported by the National Institute on Drug Abuse (F31 DA041104, T32 Training Grant “Predoctoral Training in Addiction Science”), T32 NS007431, and UNC Graduate Training Program in Translational Medicine supported by HHMI. YYIS was supported by the National Institute of Mental Health (R01 MH111429, R41 MH113252, R21 MH106939), National Institute of Neurological Disorders and Stroke (R01 NS091236), Brain and Behavior Research Foundation, and American Heart Association (15SDG23260025). GDS was supported by the Brain and Behavior Research Foundation, the Foundation of Hope, the Klarman Family Foundation, and the National Institute on Drug Abuse (R01 DA032750, and R01 DA038168).References
1. Di Chiara, G. & Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988: 85(14): 5274-5278.
2. Decot HK, Namboodiri VM, Gao W, McHenry JA, Jennings JH, Lee SH, Kantak PA, Jill Kao YC, Das M, Witten IB, Deisseroth K, Shih YI, Stuber GD. Coordination of Brain-Wide Activity Dynamics by Dopaminergic Neurons. Neuropsychopharmacology. 2016: 1-13.
3. Albaugh DL, Salzwedel A, Van Den Berge N, Gao W, Stuber GD, Shih YY. Functional Magnetic Resonance Imaging of Electrical and Optogenetic Deep Brain Stimulation at the Rat Nucleus Accumbens. Scientific Reports. 2016: 6(31613): 1-13.
4. Wolf, ME. Addiction:Making the connection between behavioral changes and neuronal plasticity in specific pathways. Molecular Inverventions. 2002: 2(3): 147-157.
5. Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008: 454(7200): 118-121.
Figures