4090
Application of multiphase pseudo-continuous ASL for quantitative blood flow measurement in rat brain metastasis1CRUK and MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom, 2Institute of Biomedical Engineering, Department of Engineering, University of Oxford, Oxford, United Kingdom
Synopsis
Arterial spin labelling (ASL) MRI is a useful clinical method of measuring blood flow in brain disorders such as tumours. This work presents a pre-clinical assessment of cerebral blood flow (CBF) by pseudo-continuous ASL in progressing rat breast cancer brain metastases. A statistically significant decrease in CBF of brain metastases was readily observed when tumours had grown sufficiently to breach the blood brain barrier, allowing gadolinium enhancement in T1-weighted images. Upon histological analysis, the brain metastases proved to be hypoxic, consistent with a reduction in CBF in those regions.
Purpose
Arterial spin labelling (ASL) MRI has numerous potential applications in the clinic. In 2015, a white paper with guidelines for standardising ASL-MRI in the clinic was published1. Similar standardisation, however, is still lacking in pre-clinical MRI, with many laboratories performing ASL on rodents and publishing a range quantitative cerebral blood flow values. Optimised parameters for ASL in rats were previously determined by using a multiphase pseudo-continuous ASL (pCASL) in three strains of naïve rats. Here, our aim was to determine how these optimised pCASL approach could be used to measure perfusion changes occurring in tumours in a rat model of brain metastasis.Methods
Female Berlin-Druckrey IX (BD-IX) rats were injected in the left striatum with 1000 ENU1564 (N-ethyl-N-nitrosourea-induced rat mammary adenocarcinoma) cells in 1 µL PBS, or with PBS alone as control. MRI experiments were performed on isoflurane anaesthetised rats at weeks 2, 3, and 4 post-tumour cell injection using a 9.4 T MRI spectrometer (Agilent) with a 72 mm volume transmit coil and a 4-channel surface receive array (Rapid Biomedical). Pre- and post-gadolinium T1-weighted along with T2-weighted anatomical (FSEMS) multi-slice images were acquired for each animal (FOV=32x32 mm, slice thickness=1 mm, 256x256 matrix). Additionally, T1 and T2 maps were acquired, together with multiphase2 pCASL data in the same slice pattern (single-shot spin echo EPI, FOV=32x32 mm, 64x64 matrix, slice thickness=1 mm). For pCASL, a 6.2 mm wide tagging plane was placed perpendicular to the carotid arteries with a labelling bolus duration of 1.4 s (TR=4 s, TE=28.7 ms; Figure 1). Post label delays used for quantitative pCASL were either 0.55 s or 0.65 s at later time points in rats injected with ENU cells to account for tortuosity of tumour vessels and possible delayed blood arrival times. Multiphase pCASL used radiofrequency (RF) pulses with eight phase increments between 0˚ and 315˚, in steps of 45˚ (4.4 µT, FA=40˚). pCASL data from eight phases were fit to a modified Fermi function with α=66 and β=21 using a modified version of BASIL3 to get cerebral blood flow (CBF) maps. Immunohistochemistry targeting the endothelial cell marker CD31 (vessels) and pimonidazole (hypoxia) was performed on 10 µm brain cryo-sections of metastasis-bearing rats for tumour characterisation.Results
Metastatic tumours were barely visible on T2-weighted images of metastasis-bearing rats 2 weeks post-injection, but were quite evident from week 3 (Figure 2B). Similarly, blood-brain barrier breakdown occurred between weeks 2 and 3 post-tumour cell injection, as demonstrated by hyperintense regions on post-gadolinium T1-weighted images (Figure 2C). At this time, a significant decrease in CBF (Figures 2A & 3) was evident within the tumour with respect to both PBS injected controls and the contralateral striatum in ENU-injected rats. The tumour core showed the greatest decrease in CBF and histologically was found to be hypoxic in regions distant to blood vessels (Figure 4).Discussion
The significant decrease in tumour perfusion from week 3 is indicative of dysregulated blood flow within the tumour. The elevated presence of pimonidazole in areas further from CD31 stained vessels within brain metastases provides evidence of hypoxia within these tumours, reflecting the decreased CBF. The low tumour CBF values present in the metastases at a later stage are similar to the findings of Silva and colleagues in a rat primary brain tumour (glioma) model using continuous ASL4.Conclusion
With our optimised pCASL methodology, we obtained reproducible CBF values in tumour cell and PBS injected rats. The metastatic tumours proved to be hypoxic and this correlated with reduced CBF observed using multiphase pCASL. CBF values in normal brain are in line with gold-standard autoradiography measurements of CBF.Acknowledgements
This work was funded by the CRUK/EPSRC Cancer Imaging Centre (grant number C5255/A16466) in Oxford, Cancer Research UK (grant number C5255/A15935), a Medical Research Council studentship (MC_ST_U13080) and MRC supplementary award (MR/K501256/1). The authors thank James A. Meakin and Peter Jezzard (FMRIB, Oxford) for their pCASL MRI sequence, which was modified for use on the Agilent system.References
1 Alsop, D. C., Detre, J. A., Golay, X., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine 73, 102-116, doi:10.1002/mrm.25197 (2015).
2 Jung, Y., Wong, E. C. & Liu, T. T. Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magnetic Resonance in Medicine 64, 799-810, doi:10.1002/mrm.22465 (2010).
3 Chappell, M. A., Groves, A. R., Whitcher, B. & Woolrich, M. W. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Transactions on Signal Processing 57, 223-236, doi:10.1109/tsp.2008.2005752 (2009).
4 Silva A.C., Kim S.G., Garwood M. Imaging Blood Flow in Brain Tumors Using Arterial Spin Labeling. Magnetic Resonance in Medicine. 44, 169-173, doi: 10.1002/1522-2594(200008)44:2<169::AID-MRM1>3.0.CO;2-U (2000).
Figures
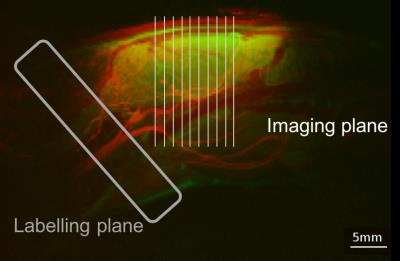
Positioning of labelling and imaging plane in rat pCASL.
T2-weighted sagittal anatomical scan of rat head (green) superimposed with a maximal intensity Z-projection of time of flight angiography (red) demonstrating the positioning of the labelling plane (grey) and of the imaging slices (white).
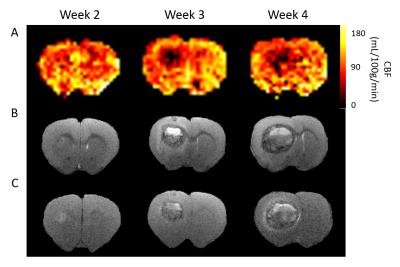
Metastatic tumour progression.
A CBF maps from multiphase pCASL at weeks 2, 3, and 4. B T2-weighted and post-gadolinium T1-weighted (C) scans of same brain slice.
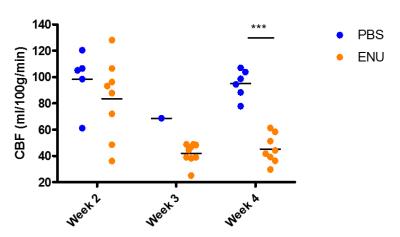
CBF of metastases in rat brain compared with PBS-injected control rats.
Tumour region of interest (ROI) was drawn on anatomical images in ENU-injected rats and representative ROIs were selected in PBS-injected rats. Each dot represents the average of the ROI in one animal. (***p<0.001)
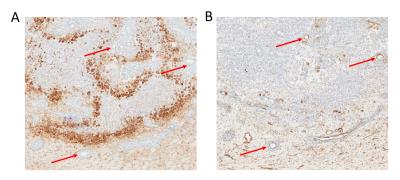
Immunohistochemical analysis of brain metastases 3-weeks post tumour cell injection.
A 10 µm brain section of ENU-injected rat stained with pimonidazole antibody, staining hypoxic tissue. B Consecutive 10 µm brain section of ENU-injected rat stained with CD31 antibody, showing vessels in brown staining. Red arrows represent vessels visible on both brain sections.