4089
Correlation of striatal remodeling with changes in song performance: a longitudinal diffusion tensor imaging study of adult male zebra finchesJulie Hamaide1, Kristina Lukacova1,2, Lubica Kubikova2, Marleen Verhoye1, and Annemie Van der Van der Linden1
1Department of Biomedical Sciences, Bio-Imaging Lab, University of Antwerp, Wilrijk, Belgium, 2Institute of Animal Biochemistry and Genetics, Slovak Academy of Sciences, Bratislava, Slovakia
Synopsis
In vivo diffusion tensor neuroimaging of adult male zebra finches was used to longitudinally monitor the effects of bilateral neurotoxic lesioning of a striatal component of the song control network and to explore possible causal relationships between the observed neuroplastic changes and specific alterations in song performance.
Purpose
Convincing similarities exist between human speech and bird song learning, making songbirds a valuable model to study particular aspects of human speech in biomedical research [1]. A recent study has shown that bilateral lesioning of the striatal component of the song control circuitry induces alterations in singing behavior that coincided with the onset of striatal tissue regeneration [2]. However, it is still not clear whether additionally other brain areas are affected by the neurotoxic lesion. Therefore, the current study uses in vivo Diffusion Tensor Imaging (DTI) to trace structural neuroplastic changes over time with voxel-wise statistical analysis of the entire brain and correlate these changes with song performance of the same birds.Methods
All imaging procedures were performed on a 7 T MR system equipped with a 400 mT/m gradient insert. The zebra finches (Taeniopygia guttata; n=14) were anaesthetized with isoflurane (induction 2.5%; maintenance: 1.4-1.6 %) while respiration rate and temperature were kept within physiological ranges ((40.0 ± 0.2)°C). First, a baseline imaging session was performed which consisted of a DTI and 3D T2-weighted RARE scan. The imaging parameters are: DTI (TR: 7000ms; TE: 22ms; δ: 4ms; Δ: 12ms; b: 670s/mm²; 60 diffusion gradient directions; spatial resolution: (0.19x0.19x0.24)mm³); 3D RARE (TR: 2500ms; TEeff: 55ms; RARE factor: 8; matrix (228x256x142) with voxel resolution (0.07x0.07x0.07)mm³). Shortly after the baseline measurement, all birds received a stereotactic injection of ibotenic acid (138nl) in the left and right Area X. Two days after induction of neurotoxic lesion, a T2-weighted 3D RARE scan was obtained to verify the location of the lesion. Follow-up MRI scans were acquired 2 weeks, 1 month, 2 months, 3 months and 4 months after surgery. The 3D RARE scans were used to create a study-specific template [3]. All DTI datasets were spatially normalized to this population-based template to enable voxel-wise statistical analyses on the smoothed DTI parameter maps (i.e. fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD)) in SPM12 (Diffusion II toolbox). Two days before each MRI session, the song of the birds was recorded and analyzed off-line [4]. Song motif length, entropy, pitch and mean frequency were quantified on 20 songs per time point for each bird.Results & Discussion
Examination of the 3D RARE scan acquired 2 days after surgery revealed that 12 out of 14 birds displayed a successful lesion co-localized with Area X (Fig. 1). Song analyses indicated that motif length increased acutely after neurotoxic lesion, after which it gradually decreased towards 4 months post-surgery (Fig. 2). Importantly, the increase and decrease in motif length are due to resp. a prolongation and a shortening of the inter-syllable interval which implies that the birds first sing slower after which they recuperate and even sing slightly faster, in line with [2]. A voxel-wise repeated-measures ANOVA of the DTI parameter maps detected a significant main effect of time in FA co-localized with Area X, the damaged area, and the entry of the needle, reflecting mechanical damage to the tissue. MD and AD showed significant differences near the dorsolateral nucleus of the anterior thalamus (DLM), the commissura posterior and the cerebellum (Fig. 3). Overall, the data suggest that most microstructural adaptations take place beyond the acute phase i.e. within the first two months after neurotoxic lesion, with peak differences when comparing DTI data obtained 1 and 2 months after surgery. A voxel-wise multiple regression of the DTI parameters and song performance showed a positive correlation between motif length and MD, AD and RD in several clusters situated in caudal striatum adjacent to Area X and several rostro-dorsal areas of the meso- and nidopallium.Conclusion
This study shows that damage to one component of the song control circuitry induces structural neuroplastic adaptations in its downstream targets i.e. DLM and the cerebellum. Additionally, motif length which reflects ‘singing speed’, was positively correlated with diffusion metrics in several brain areas among which the caudal striatum immediately adjacent to the damaged area.Acknowledgements
This research was supported by grants from the Hercules Foundation (Grant Nr AUHA0012), Interuniversity Attraction Poles (IAP) (‘PLASTOCINE’: P7/17) and the research foundation –Flanders (Grant Nr G044311N) to Annemie Van der Linden. Julie Hamaide is a PhD student funded by the University of Antwerp.References
[1] Brainard and Doupe, 2013 Annu Rev Neurosci
[2] Kubikova et al. 2014 Scientific Report
[3] Avants et al., 2011 NeuroImage
[4] Tchernichovski et al. 2000 Animal Behaviour
Figures
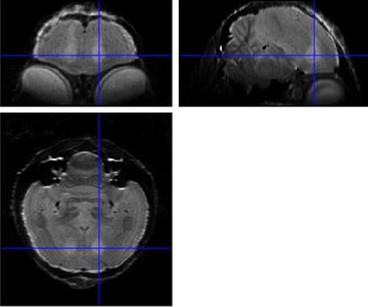
Figure 1: Average 3D RARE of all birds acquired 2 days after neurotoxic surgery to
check position of lesion (hyperintense region indicated by crosshairs).
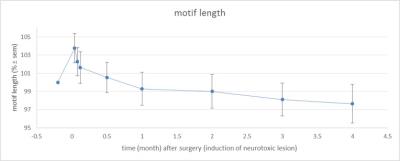
Figure 2: Motif length over time (% normalized to baseline ± SEM; baseline is 100
%).
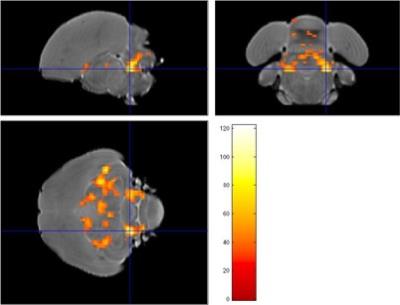
Figure 3: Statistical map (F test) comparing AD data obtained 1 and 2 months after
surgery, the crosshair points to a cluster in the cerebellum.