4085
Diffusion Tractography Reveals Pervasive Asymmetry of Cerebral White Matter Tracts in the Bottlenose Dolphin1Scripps Institution of Oceanography, University of California San Diego, San Diego, CA, United States, 2Radiology, University of California San Diego, San Diego, CA, United States, 3National Marine Mammal Foundation, San Diego
Synopsis
Introduction
Methods
The
specimen was a formalin-fixed brain of a captive 27-year-old male bottlenose
dolphin. The brain was removed and fixed
within 3 hours of death. Data was acquired using a GE 3.0 T Signa 750 MRI. DTI
was acquired in the axial plane using single-shot EPI, 60 direction diffusion-encoding,
b value 3000 s/mm2,
six non-diffusion weighted images (b0), slice thickness 3 mm,
TR 8 s, TE 82 ms, 4 averages, matrix 128 × 128 mm, FOV 200 mm, 56 axial slices,
and voxel size 0.78 × 0.78 × 3 mm. 3 Nex. Total scan time 105 minutes. Axial T2
anatomical images were also acquired. DTI
data was concatenated and eddy currents corrected using FSL. Data were fit to a diffusion model for
each voxel using the FMRIB Diffusion Toolbox13 (FDT). FA, MD,
AD, and RD maps were calculated. The FA and the main
eigenvector maps were imported into DtiStudio for fiber tracking analysis.
Fiber tracking was performed using the FACT method14. Tracking was
terminated when the local FA fell below the FA threshold of 0.1, or when the
tract-turning angle exceeded the angular threshold of 55o. A
multiple ROI approach was used to reconstruct cerebral white matter tracts using
FA maps. Eight white matter tracts were reconstructed. Measurements of the volume, voxel size15, fiber number16,
mean fiber length16, FA , MD , AD, and RD17
were acquired for each tract. Asymmetries of each tract-specific measurement
were assessed by calculating the lateralization index (LI)18. Calculations
of the relative volume and relative fiber number were performed for each tract
to determine the percentage of the total volume or total fiber number occupied
by the left and right tracts.
Results
The
anterior thalamic radiation, arcuate fasciculus, cingulum, corticocaudate
tract, external capsule, forceps minor of the corpus callosum, fornix, superior
longitudinal fasciculus system, and the sub-tracts of the superior longitudinal
fasciculus system (SLF I, SLF II, and SLF III) were reconstructed. Asymmetries
were found for the relative volume relative fiber number, and lateralization
index for most of the tracts examined (see figures 2 and 3). The absence of
lateralization for FA, MD, AD, and RD in all
of the tracts examined suggests that the tract-specific measurements were not
confounded by these parameters, and were indeed asymmetric in certain tracts.
Moreover, symmetry of microstructural diffusion parameters and uniformity of
the structural dataset indicate that macrostructural asymmetries were not due
to tissue damage or incomplete fixation of the specimen.
Discussion and Conclusion
These findings
suggest widespread structural asymmetries of cerebral white matter in this dolphin,
and provide support for the hypothesis that large brains should exhibit
pronounced lateralization. Moreover, the sparse reconstruction of the corpus
callosum in parallel with various reports on the diminutive size of the
cetacean corpus callosum relative to the volume of the cerebral hemispheres19-24 correspond to observations and predictions of reduced
interhemispheric connectivity with brain enlargement. The observation of this
pervasive asymmetry in cerebral white matter architecture is proposed to
reflect differential perception, processing, and production of social and
nonsocial sensory signals and motor actions in the bottlenose dolphin.
Acknowledgements
No acknowledgement found.References
1. Rogers LJ, Andrew R (2002) Comparative vertebrate lateralization.
Cambridge University Press
2. Ringo JL (1991) Neuronal
interconnection as a function of brain size. Brain Behav Evol 38:1–6. doi:
10.1159/000114375
3. Ringo JL, Doty RW, Demeter S, Simard PY
(1994) Time is of the essence: a conjecture that hemispheric specialization
arises from interhemispheric conduction delay. Cereb Cortex 4:331–343. doi:
10.1093/cercor/4.4.331
4. Pilleri G, Gihr M (1970) The central
nervous system of the mysticete and odontocete whales. In: Investigations on
Cetacea. pp 87–135
5. Ridgway SH (2002) Asymmetry and
symmetry in brain waves from dolphin left and right hemispheres: some
observations after anesthesia, during quiescent hanging behavior, and during
visual obstruction. Brain Behav Evol 60:265–274. doi: 10.1159/000067192
6. Ridgway SH, Brownson RH (1984)
Relative brain sizes and cortical surface areas in odontocetes. Acta Zool Fenn
172:149–152.
7. Ridgway SH, Carder DA (1990) Tactile
sensitivity, somatosensory responses, skin vibrations, and the skin surface
ridges of the bottlenose dolphin, Tursiops truncatus. In: Sensory
Abilities of Cetaceans. Springer, pp 163–179
8. Supin AY, Mukhametov LM, Ladygina TF, et al (1978)
Electrophysiological studies of the dolphin’s brain
9. Goley PD (1999) Behavioral aspects of sleep in
Pacific white-sided dolphins (Lagenorhynchus obliquidens, Gill 1865).
Mar Mamm Sci 15:1054–1064. doi: 10.1111/j.1748-7692.1999.tb00877.x
10. Rattenborg NC, Amlaner CJ, Lima SL (2000)
Behavioral, neurophysiological and evolutionary perspectives on unihemispheric
sleep. Neurosci Biobehav Rev 24:817–842. doi: 10.1016/S0149-7634(00)00039-7
11. Ridgway SH, Carder D, Finneran J, et al (2006a)
Dolphin continuous auditory vigilance for five days. J Exp Biol 209:3621–3628.
doi: 10.1242/jeb.02405
12. Lyamin OI, Manger PR, Ridgway SH, et al (2008)
Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav R 32:1451–1484.
doi: doi:10.1016/j.neubiorev.2008.05.023
13. Behrens TEJ, Woolrich MW, Jenkinson M, et al (2003)
Characterization and Propagation of Uncertainty in Diffusion-Weighted MR
Imaging. Magn Reson Med 50:1077–1088. doi: 10.1002/mrm.10609
14. Mori S, Crain BJ, Chacko VP, van Zijl PCM (1999)
Three-dimensional tracking of axonal projections in the brain by magnetic
resonance imaging. Ann Neurol 45:265–269. doi:
10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3
15. Hagmann P, Cammoun L, Martuzzi R, et al (2006) Hand
preference and sex shape the architecture of language networks. Hum Brain Mapp
27:828–835. doi: 10.1002/hbm.20224
16. Jiang H, van Zijl PCM, Kim J, et al (2006)
DtiStudio: resource program for diffusion tensor computation and fiber bundle
tracking. Comput Meth Prog Bio 81:106–116. doi: http://dx.doi.org/10.1016/j.cmpb.2005.08.004
17. Beaulieu C (2014) The biological basis of diffusion
anisotropy. In: Johansen-Berg H, Behrens TEJ (eds) Diffusion MRI: From
quantitative measurement to in-vivo neuroanatomy, 2nd edn. Elsevier Academic
Press, pp 155–183
18. Vernooij M, Smits M, Wielopolski P, et al (2007)
Fiber density asymmetry of the arcuate fasciculus in relation to functional
hemispheric language lateralization in both right- and left-handed healthy
subjects: A combined fMRI and DTI study. Neuroimage 35:1064–1076. doi:
10.1016/j.neuroimage.2006.12.041
19. Tarpley RJ, Ridgway SH (1994) Corpus callosum size
in delphinid cetaceans. Brain Behav Evol 44:156–165. doi: 10.1159/000113587
20. Keogh MJ, Ridgway SH (2008) Neuronal fiber
composition of the corpus callosum within some odontocetes. Anat Rec 291:781–789.
doi: 10.1002/ar.20701
21. Montie EW, Schneider G, Ketten DR, et al (2008)
Volumetric neuroimaging of the Atlantic white-sided dolphin (Lagenorhynchus
acutus) brain from in situ magnetic resonance images. Anat Rec 291:263–282.
doi: 10.1002/ar.20654
22. Manger PR, Hemingway J, Haagensen M, Gilissen E
(2010) Cross-sectional area of the elephant corpus callosum: comparison to
other eutherian mammals. Neurosci 167:815–824. doi:
10.1016/j.neuroscience.2010.02.066
23. Berns GS, Cook PF, Foxley S, et al (2015) Diffusion
tensor imaging of dolphin brains reveals direct auditory pathway to temporal
lobe. P Roy Soc B 282:20151203. doi: http://dx.doi.org/10.1098/rspb.2015.1203
24. Wright A, Scadeng M, Stec D, et al (2016)
Neuroanatomy of the killer whale (Orcinus orca): a magnetic resonance
imaging investigation of structure with insights on function and evolution.
Brain Struct Funct 1–20. doi: 10.1007/s00429-016-1225-x
Figures
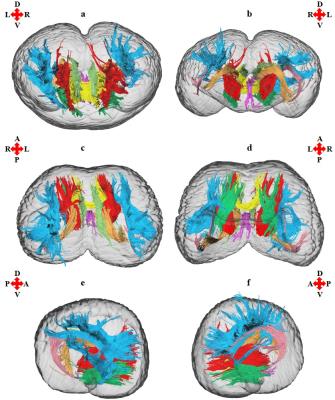
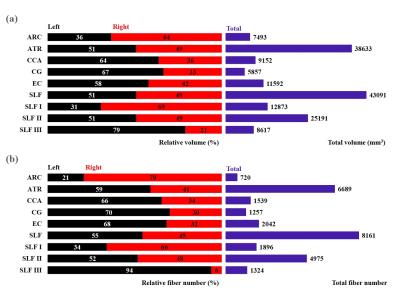
Fig. 2 (a) Total volume (mm3, purple) and relative volume
(%) for each tract (left, black; right, red) and (b) total fiber number (purple)
and relative fiber number (%) for each tract (left, black; right, red). Left
and right tracts combined represent 100% of the total volume or total fiber
number. ARC (arcuate fasciculus), ATR (anterior thalamic radiation), CCA
(corticocaudate tract), CG (cingulum), EC (external capsule), SLF (superior
longitudinal fasciculus system), SLF I (superior longitudinal fasciculus I), SLF
II (superior longitudinal fasciculus II), SLF III (superior longitudinal
fasciculus III).
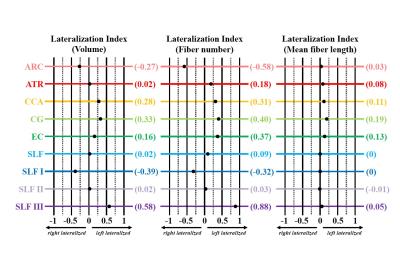
Fig. 3 Lateralization index (LI) for the volume, fiber number, and mean fiber length of the arcuate fasciculus (ARC, rose) anterior thalamic radiation (ATR, red), corticocaudate tract (CCA, orange), cingulum (CG, light green), external capsule (EC, dark green), superior longitudinal fasciculus system (SLF, light blue), superior longitudinal fasciculus I (SLF I, dark blue), superior longitudinal fasciculus II (SLF II, light purple), and superior longitudinal fasciculus III (SLF III, dark purple). Tract-specific LI values for each measurement are shown in parentheses on the right.