4084
MRI monitoring of the in vivo permeability increase of brain tumor vessels induced by synchrotron microbeam radiation therapy1INSERM, Grenoble, France, 2University of Bern, Bern, Switzerland, 3INSERM, 4ESRF, 5University of Bern
Synopsis
Synchrotron microbeam radiation therapy (MRT), a spatially fractionated preclinical radiotherapy, is more efficient than broad beam irradiation (BB) at opening the blood-brain tumor barrier of intracranial rodent glioblastomas. MRT-induced increase of tumor vascular permeability is significantly greater, earlier and more prolonged than in the BB alone group, especially in highly proliferative areas. MRT targeted all tumor regions, including areas not impacted by BB. High dose microbeams might be used to facilitate the delivery of intravenously injected drugs to tumoral tissue: Adjuvant chemotherapy might thus be more effective when coupled with MRT than with homogeneous radiation fields used in conventional radiotherapy.
INTRODUCTION
Efficiency of blood-borne chemotherapies is hampered by a limited exposure of all cells of the tumor mass to a sufficient drug concentration due to the anatomic/physiologic characteristics of solid tumors 1. The challenge of drug delivery is increased particularly for brain tumors due to the presence of blood brain barrier (BBB) 2. Conventional radiotherapy was reported as an efficient strategy to overcome these physio-anatomical limitations by temporal disruption of the blood barrier in the tumor 3. Our group has previously shown that synchrotron microbeam radiation therapy (MRT), a spatially fractionated preclinical radiotherapy, increases as well vessel permeability of high grade brain tumor 4; however the efficacy of the two radiotherapies was never confront until now.
In the present work, we used magnetic resonance imaging (MRI) to compare the blood tumor barrier permeability changes induced by MRT with a spatially non-fractionated radiotherapy. Vessel permeability (assessed as dynamic contrast enhancement, DCE) was monitored in normal brain tissue and in the whole tumor volume. In addition to find out whether tissue characteristics influence the use of a vascular permeability window for an adjuvant systemic treatment, we evaluate vessel integrity in tumor regions with different cerebral blood flow (CFB), blood volume (BV), oedema (ADC) and tissue oxygenation (CRMO2) values.
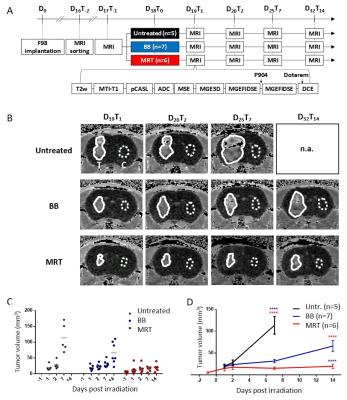
METHODS
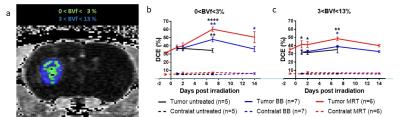
Male rats bearing intracranial highly malignant F98 gliomas were randomized into 3 groups: untreated, or exposed to MRT (peak and valley dose: 241 and 10.5 Gy respectively) or to broad beam irradiation (BB) delivered at comparable doses; both applied by two arrays, intersecting orthogonally in the tumor region. In vivo multiparametric MRI5 (tumour volume, diffusion-weighted MR imaging (ADC), blood volume fraction (BVf), cerebral blood flow (CFB), tissue oxygenation (CRMO2) and vessel wall integrity (dynamic contrast enhancement) was performed 1 day before (T-1) and 1, 2, 7 and 14 days after irradiation (T1, T2, T7 and T14). MRI sequences acquired for each animal and experimental timeline are represented Figure 1 A. Data were extracted from the whole tumor region of interest or from specific areas displaying voxels with values inferior (inf-) or superior (sup-) to a threshold defined as the rounded mean values across the tumors of our study before treatment (T-1): 1100 mm2.sec-1 for ADC, 70 ml.min-1.100g-1 of tissue for CBF, 3% for BVf and 6.5 a.u. for CMRO2.
RESULTS
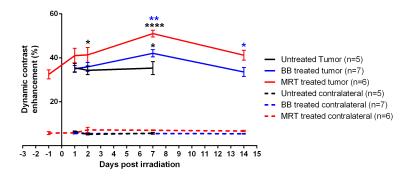
Tumor volumes measured by MRI revealed that MRT reduced tumor growth more than BB irradiation did, i.e. tumors were 3.4 times smaller at T14 (19.5 ± 11.7 mm3) after MRT than after BB (65.9 ± 34.2 mm3, p<0.0001). In the tumor, MRT and BB treatments increased the permeability over the time, with a maximum of DCE 7 days after irradiation. Further, DCEs measured in MRT-treated tumors were significantly higher than those of tumors treated by BB at T2 (+20.7%; p=0.0454), T7 (+21.3%; p=0.0064) and at T14 (+22.6%; p=0.0199); Figure 2. At the maximum increase of the permeability, i.e. 7 days post treatment for both irradiation types (Figure 2), MRT and BB resulted in different efficacies since MRT induced an higher vascular permeability in all physiologically characterized tumor areas, including areas not impacted by BB, as for example the area with the highest BVf, and hypo-oxygenated areas; areas which are in addition mainly distributed in the outer shell of the tumor mass (Figure 3 et 4).DISCUSSION
In addition to be more efficient that BB in tumor growth control, MRT does not modify the vascular permeability of normal brain tissue. MRT-induced increased tumor vascular permeability is (i) significantly greater, (ii) earlier and more prolonged than the BB counterpart, especially in highly proliferative tumor areas (iii) and it targets all tumor areas discriminated by physiological characteristics, including those areas not damaged by homogeneous irradiation.CONCLUSION
MRT leads to a significantly higher, earlier and more continuous increase in tumor blood vessel permeability than homogeneous BB irradiation, without affecting normal brain tissue. In addition, MRT induces an increase in vascular permeability in all tumor areas with particular physiological characteristics, including those tumor areas not impacted by homogeneous irradiation. Finally, we showed that MRT is more efficiently disrupts brain tumor vessels in the most actively proliferating area of the tumor considered as the relevant target area for adjuvant drug delivery. Therefore, high dose microbeams might be used to facilitate the delivery of intravenously injected drugs to tumoral tissue. An adjuvant chemotherapy might be more effective when coupled with MRT than with homogeneous radiation fields used in conventional radiotherapy.Acknowledgements
In fond memory of Régine Farion who has generously and with heartwarming enthusiasm contributed to this work during many years.
The authors thank the Bernische Krebsliga for the support via the grant n.37223, as well as the “Conseil Régional Rhône-Alpes”, la “Ligue contre le Cancer, comité de la Drome”, “l’Association pour la Recherche contre le Cancer” and Imoxy ANR for research funding. Grenoble MRI facility IRMaGe was partly funded by the French program “Investissement d’Avenir” run by the “Agence Nationale pour la Recherche”; grant “Infrastructure d’avenir en Biologie Santé” - ANR-11-INBS-0006.
References
1. Holback H, Yeo Y. Intratumoral drug delivery with nanoparticulate carriers. Pharm Res. 2011;28(8):1819-30. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3131473&tool=pmcentrez&rendertype=abstract.
2. Pollay M, Roberts PA. Blood-brain barrier: a definition of normal and altered function. Neurosurgery. 1980;6(6):675-85. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7001265.
3. van Vulpen M, Kal HB, Taphoorn MJB, El-Sharouni SY. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep. 2002;9(4):683-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12066192.
4. Bouchet A, Lemasson B, Leduc G, et al. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9L gliosarcoma vascular networks. Int J Rad Onc Biol Phys. 2010;78(5):1503-1512.
5. Lemasson B, Bouchet A, Maisin C, et al. Multiparametric MRI as an early biomarker of individual therapy effects during concomitant treatment of brain tumours. NMR Biomed. 2015;28(9):1163-1173. doi:10.1002/nbm.3357.
Figures